Show filters
Generating structured foam via flowing through a wire array
Efficient manufacturing methods could unlock foams with tailored, anisotropic properties. Conventional foam production methods rely on the self-arrangement of bubbles, typically leading to isotropic materials, or involve intricate additive layering processes. This study presents a simple, passive technique to modify the foam structure. A set of thin parallel wires ...
HPCP Summer School FHNW/PSI
The institute for Data Science of the FHNW in Brugg - Windisch and AWI department of PSI held a joint course on high performance computing. The course addressed computer science students of FHNW and interested individuals at PSI. Two full days at the FHNW (with Apero) were followed by two full days at PSI using Merlin6 (with tour).
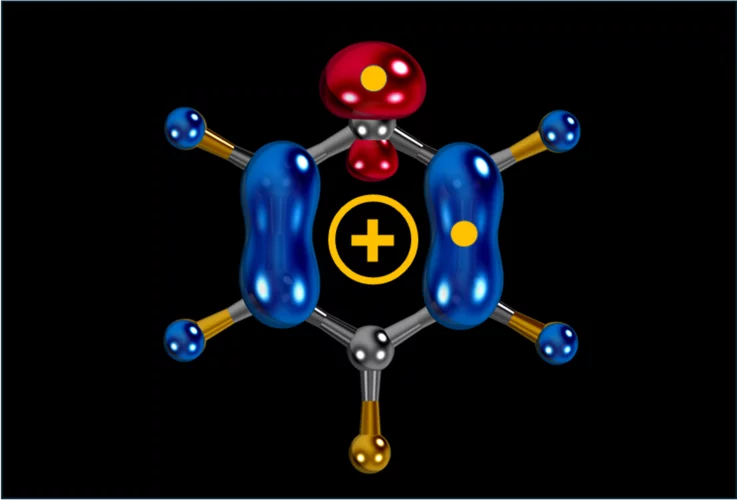
Carbocation, diradical, and superelectrophile in one molecule?
The pentafluorophenyl cation (C₆F₅⁺) breaks these rules with a borderline “crazy” reactivity.
Discovery of Nodal-Line Superconductivity in Chiral Crystals
Chiral crystals, whose key feature is the structural handedness, host exotic quantum phenomena driven by the interplay of band topology, spin-orbit coupling (SOC), and electronic correlations. Due to the limited availability of suitable chiral-crystal materials, their unconventional superconductivity (SC) remains largely unexplored.
Here, the discovery ...
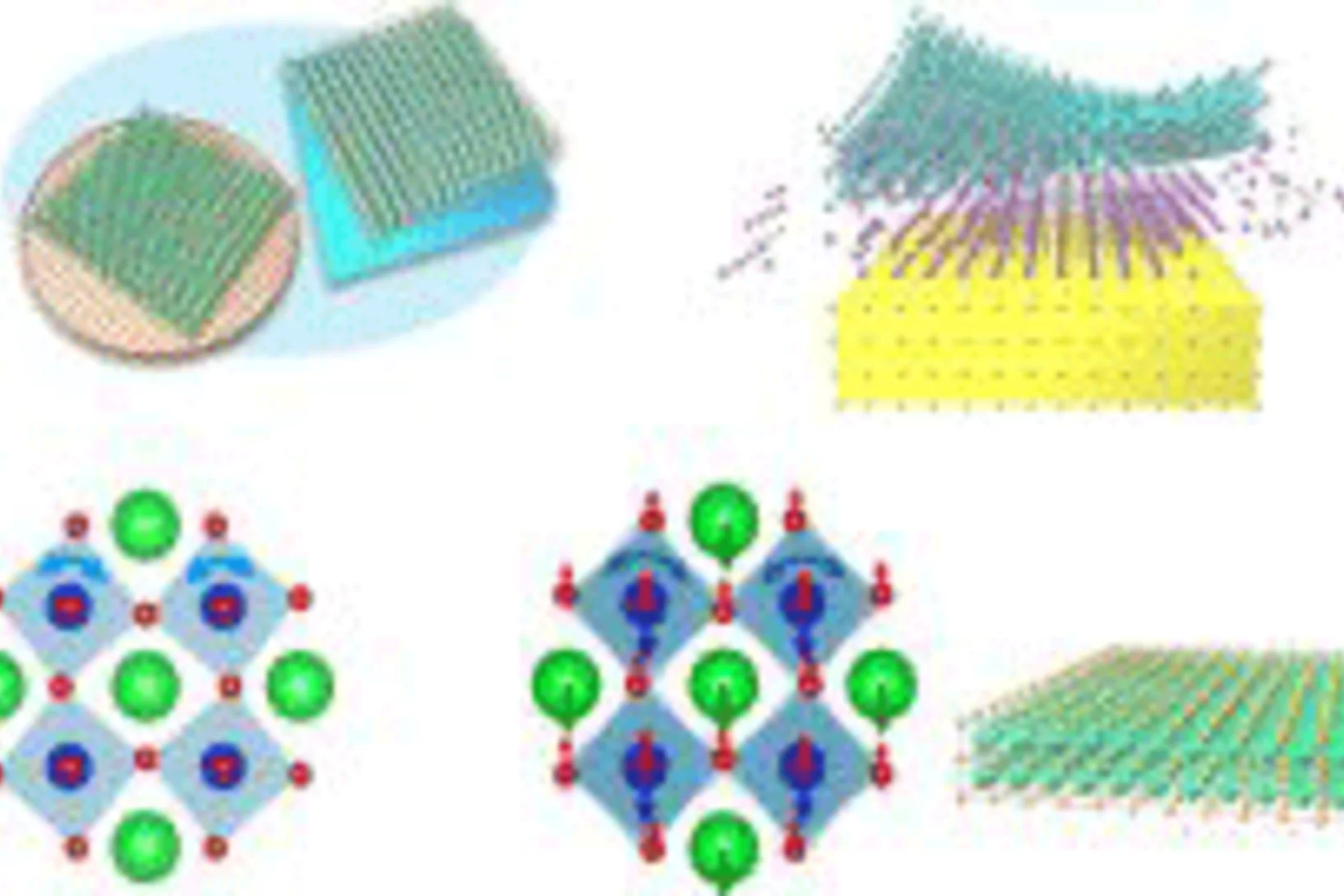
Ferroaxial density wave from intertwined charge and orbital order in rare-earth tritellurides
The discovery of the axial amplitude mode—commonly referred to as the Higgs mode—in charge density wave systems, such as rare-earth tritellurides, indicates the presence of a hidden order. A theoretical study proposed that this axial Higgs mode arises from a hidden orbital texture of the charge density wave, which produces a ferroaxial charge order.
However, experimental evidence ...
Why Ni-Cu Alloys Fail in 3D Printing
3D printing with nickel–copper alloys holds great promise — but hidden mechanisms can cause them to crack. Our latest study reveals why.
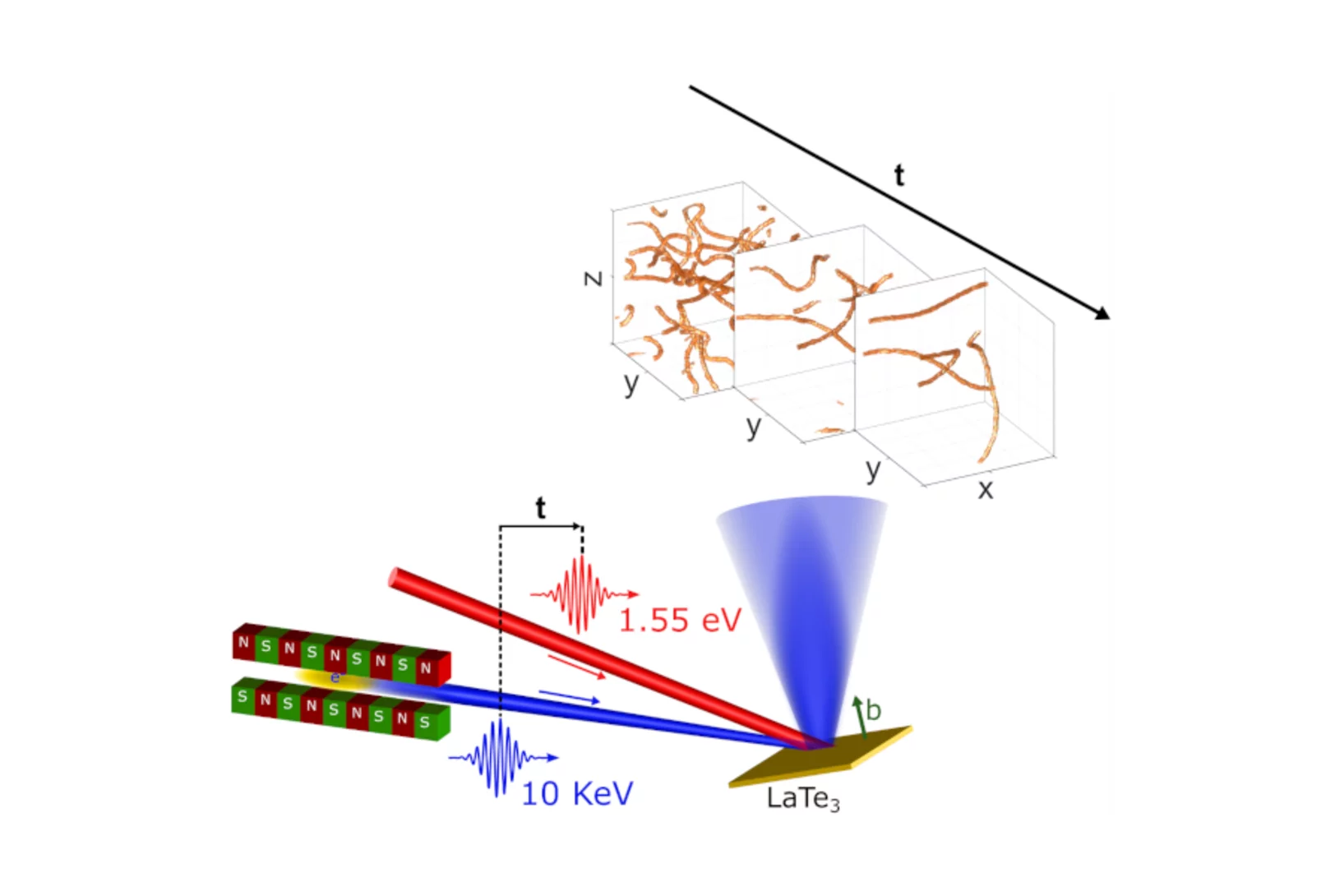
Topological defects determine evolution of charge density wave phase transition
Total scattering signals collected at SwissFEL reveal the role of topological defects when switching properties of a charge density wave material. The defect formation and dynamics after laser excitation reveals new insights into the functionality of quantum materials.
PhD student Hao Guo received the SAPhW Poster Award for Best Poster in Clinical Pharmacy at the 2025 Swiss Pharma Science Day
We congratulate Hao Guo for receiving the award of the best poster at the Swiss Pharma Day.
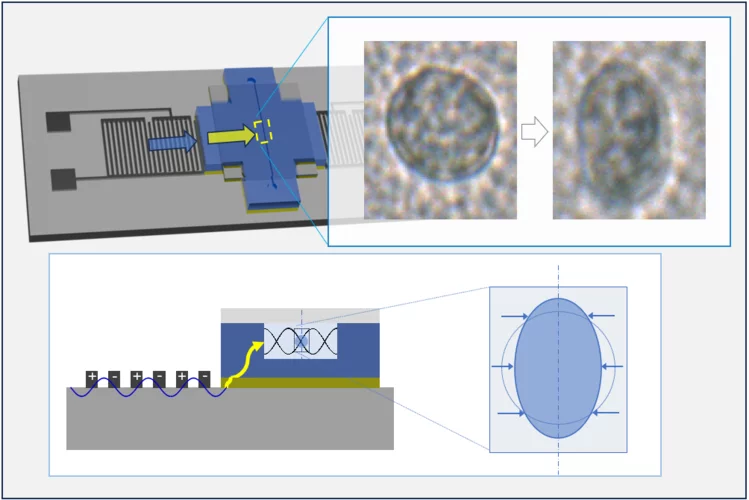
Manipulating microscopic object with sound
Manipulating microscopic objects with sound: hybrid acoustic tweezers with strong acoustic field were developed and successfully applied for transient mechanical deformation and capturing of biological cells.
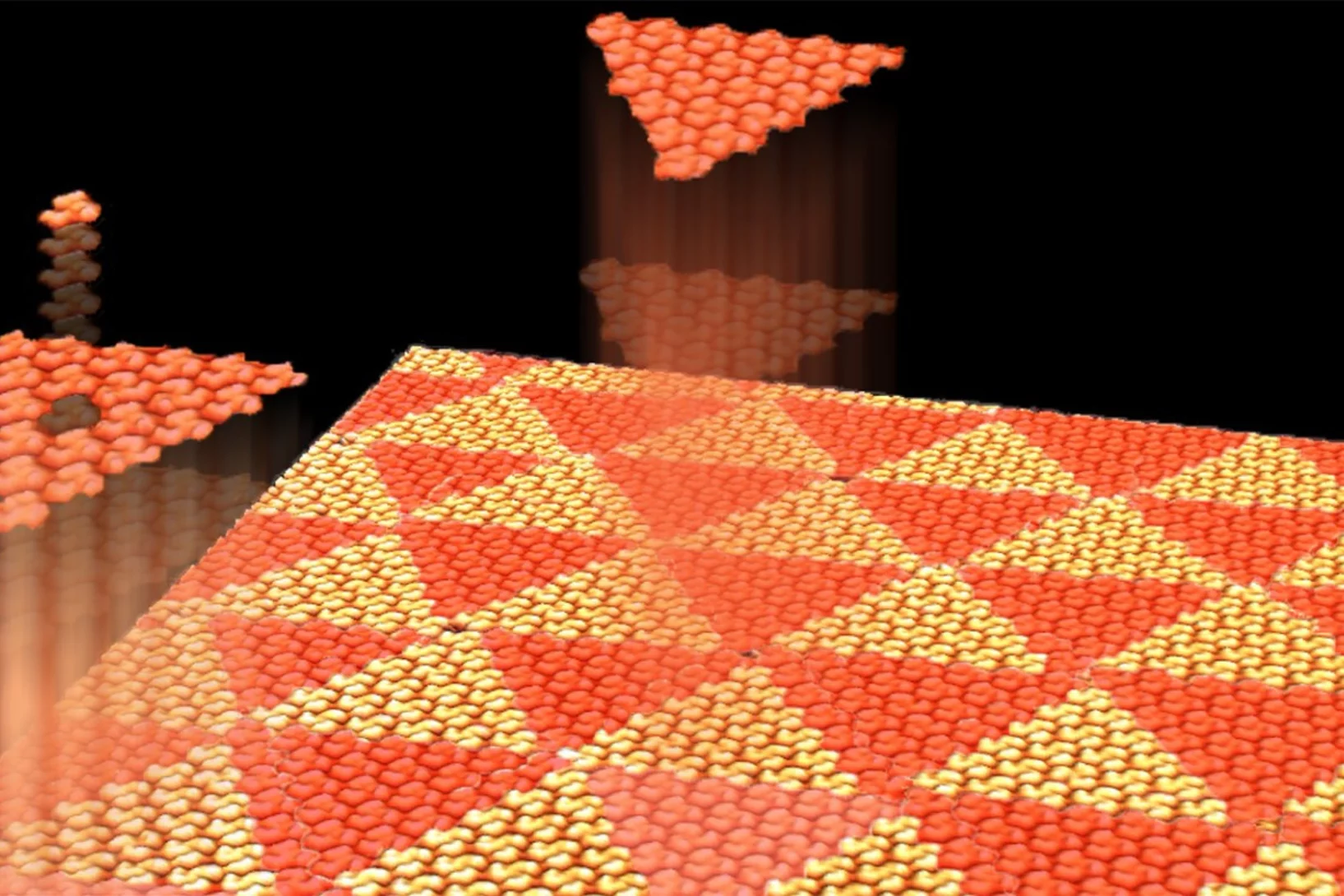
Aperiodic Chiral Tiling by Molecular Self-Assembly
The 2D self-arrangement of a molecule that resembles three-armed spirals leads to a triangular pattern that is effectively aperiodic.
Machine Learning Accelerates Alloy Design for Additive Manufacturing
A new machine learning–designed alloy demonstrates how materials can be tailored for specific performance properties required in additive manufacturing.
Observation of Magnetic Pseudogap Behavior in Phosphorus-Doped Silicon
The recent discovery of a Kondo condensate in phosphorus-doped silicon (Si:P) presents its significant potential for achieving novel many-body quantum states. Si:P exhibits Kondo condensation, characterized by an energy gap in the electronic density of states, while the precise nature of its magnetic state has yet to be determined.
Here, we utilize ...
Demystifying electron ptychography with the PtychoScopy tool
The open-source PtychoScopy tool guides users towards higher quality and faster electron ptychographic reconstructions.
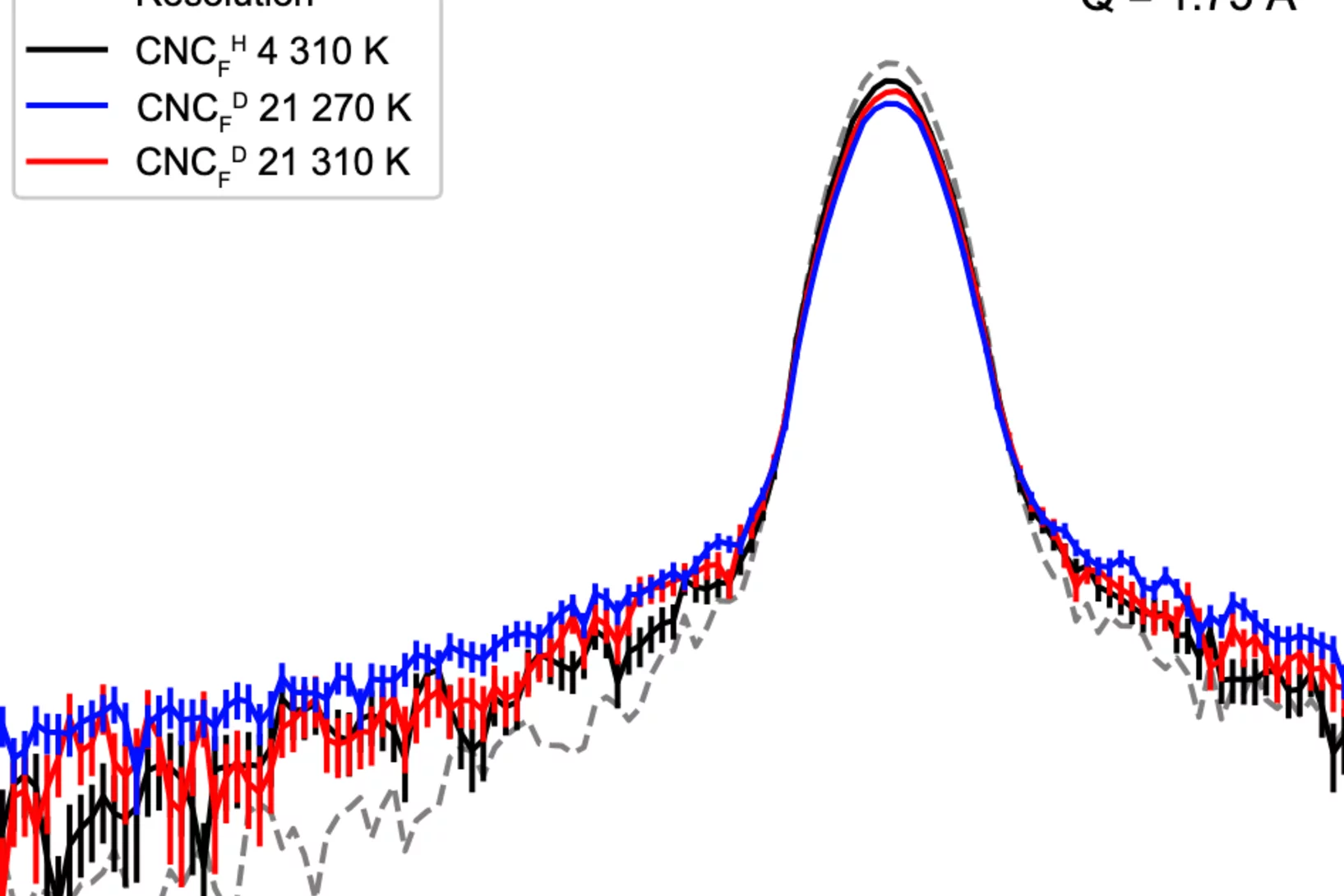
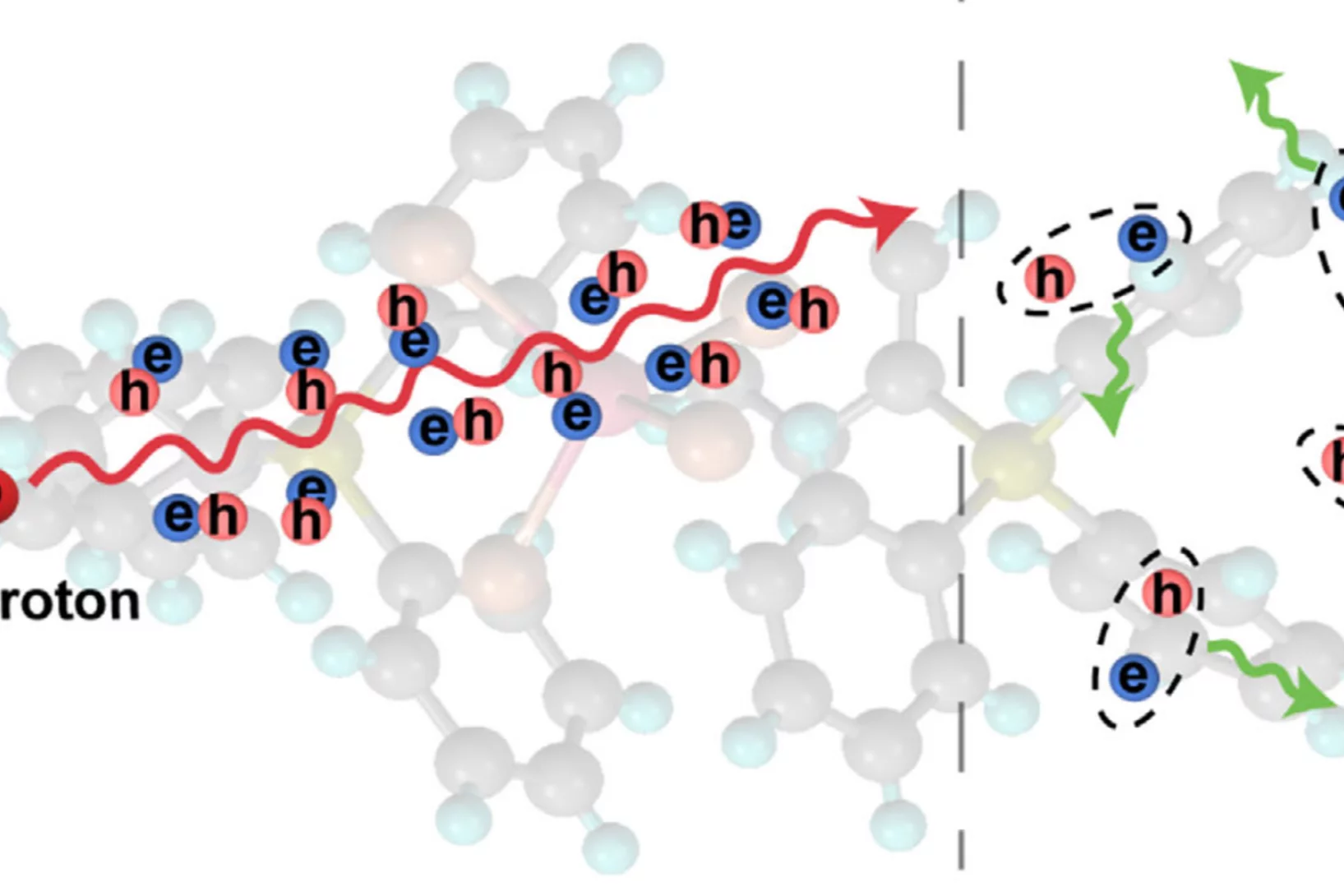
Hydration- and Temperature-Dependent Rotational Dynamics and Water Diffusion in Nanocellulose
Nanocellulose is a promising alternative to fossil-derived materials, but its development is hindered by a limited understanding of cellulose–water interactions. Herein, quasielastic neutron scattering (QENS) is used to investigate how hydration and temperature affect the localized rotations in cellulose nanocrystals (CNC) and the diffusion of mobile water. QENS reveals ...
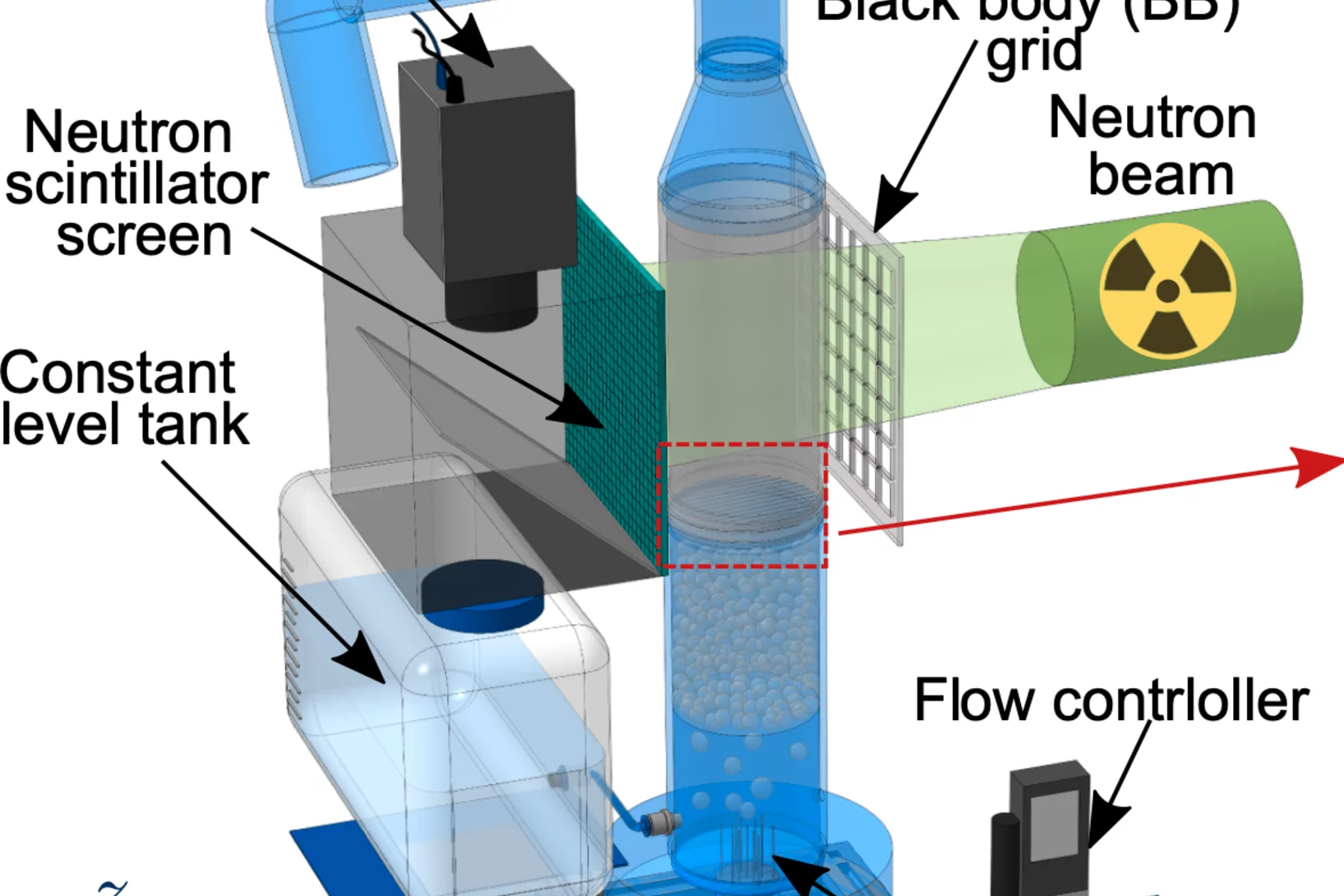
Bright Monocompound Metal Halide Scintillator for Fast Neutron Radiography
Fast neutron imaging is a promising technique for visualizing objects containing dense, mixed light-and-heavy-elements materials, such as combustion engines, nuclear fuel assemblies, and fossils, where X-rays and thermal neutrons are ineffectiv. However, the limited efficiency of current detection technologies hinders their widespread adoption. Recoil proton detection ...
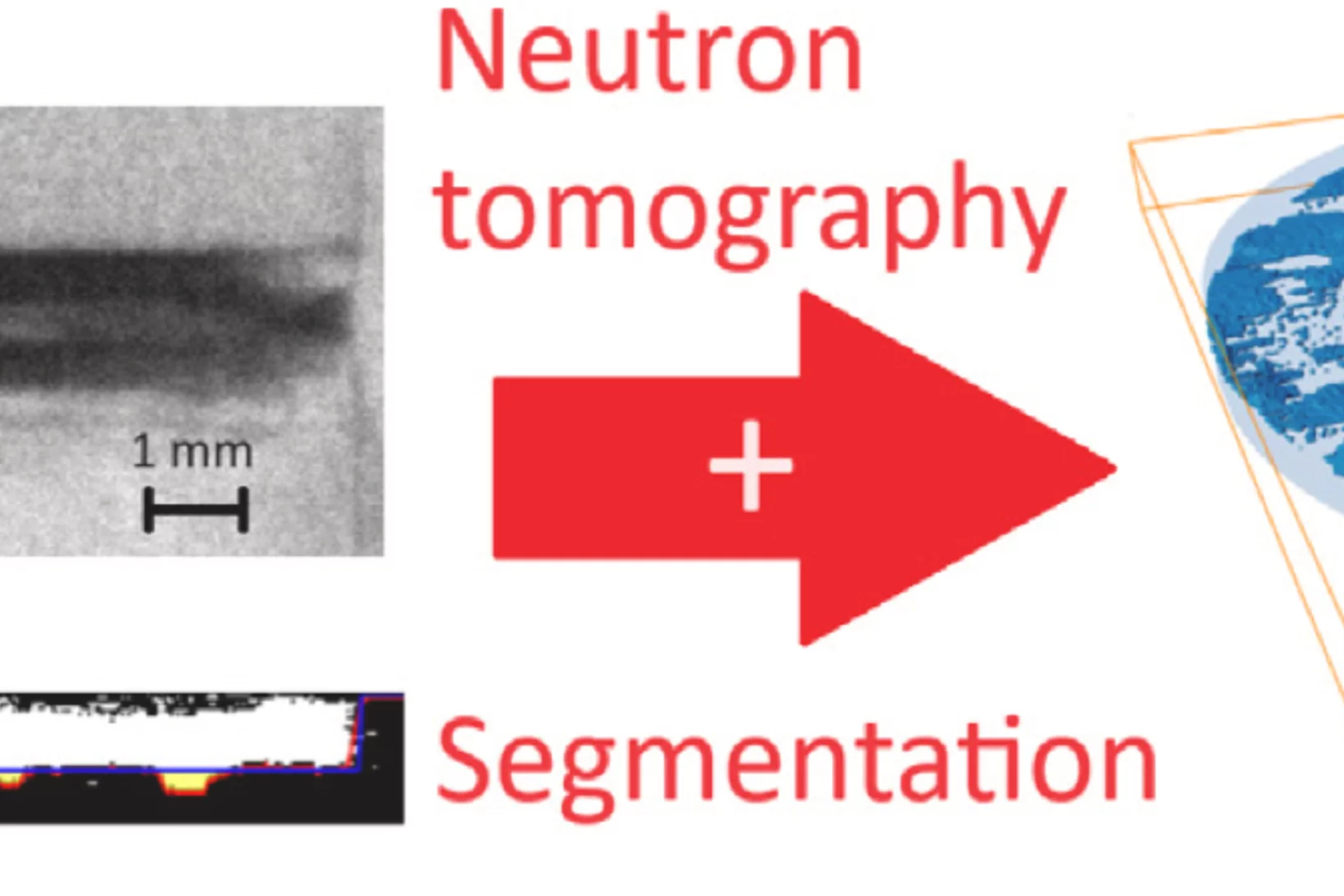
Neutron imaging in 2D and 3D as a powerful tool to investigate electrolyte degradation and plating mechanisms in sodium-ion batteries
To develop durable and high-performance sodium-ion batteries, it is crucial to understand the degradation processes taking place during electrochemical cycling. This study presents the first demonstration of visualizing the effects of electrolyte degradation in sodium-ion batteries, via 2D and 3D neutron imaging thereby visualizing the degradation of the cells. The experiment ...
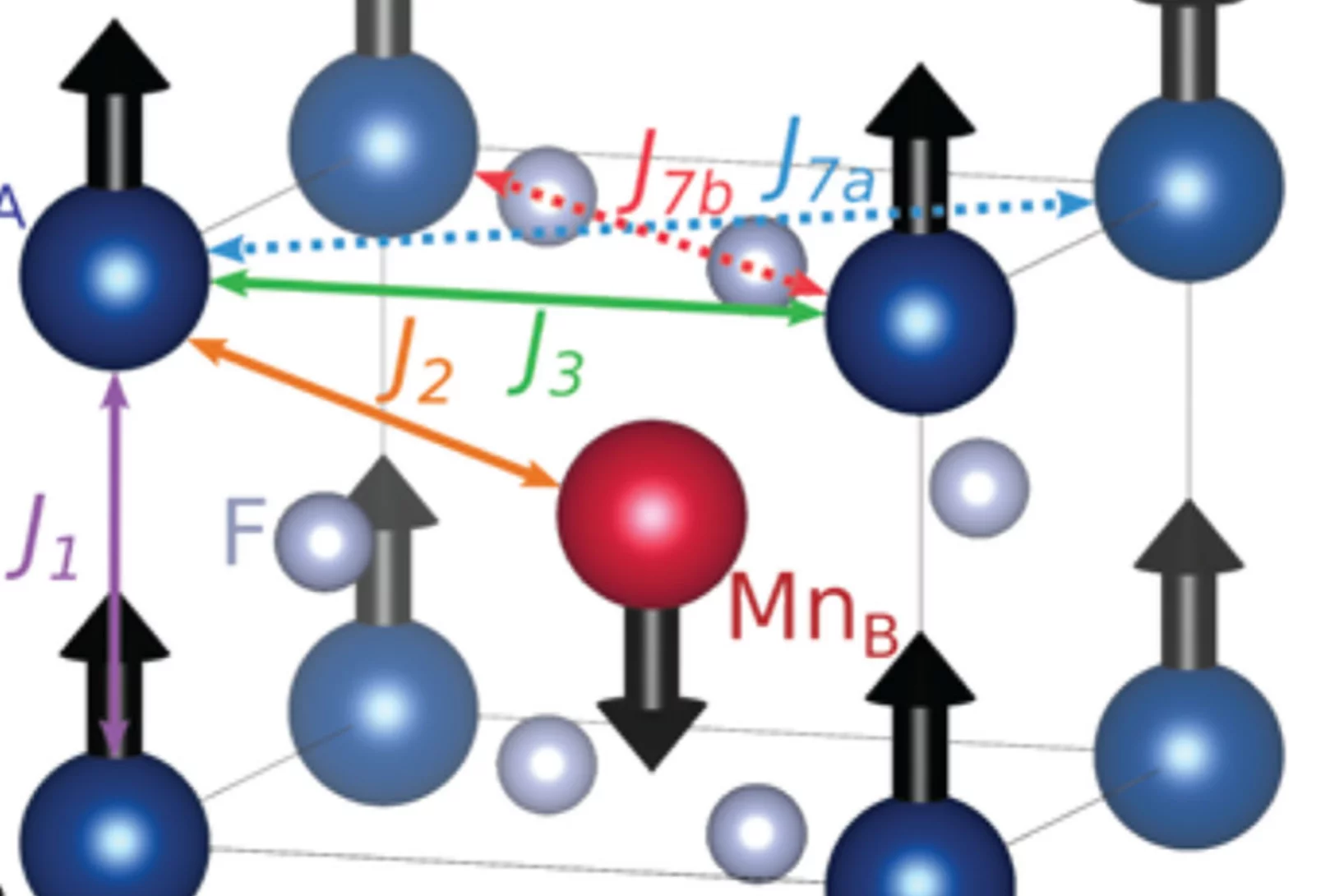
Microscopic Origin of Reduced Magnetic Order in a Frustrated Metal
Although magnetic frustration in metals provides a promising avenue for novel quantum phenomena, their microscopic interpretation is often challenging. Here, we use the face-centered cubic intermetallic HoInCu4 as model material to show that Hamiltonians neglecting the charge degree of freedom are appropriate for frustrated metals possessing low density of states at the Fermi surface ...
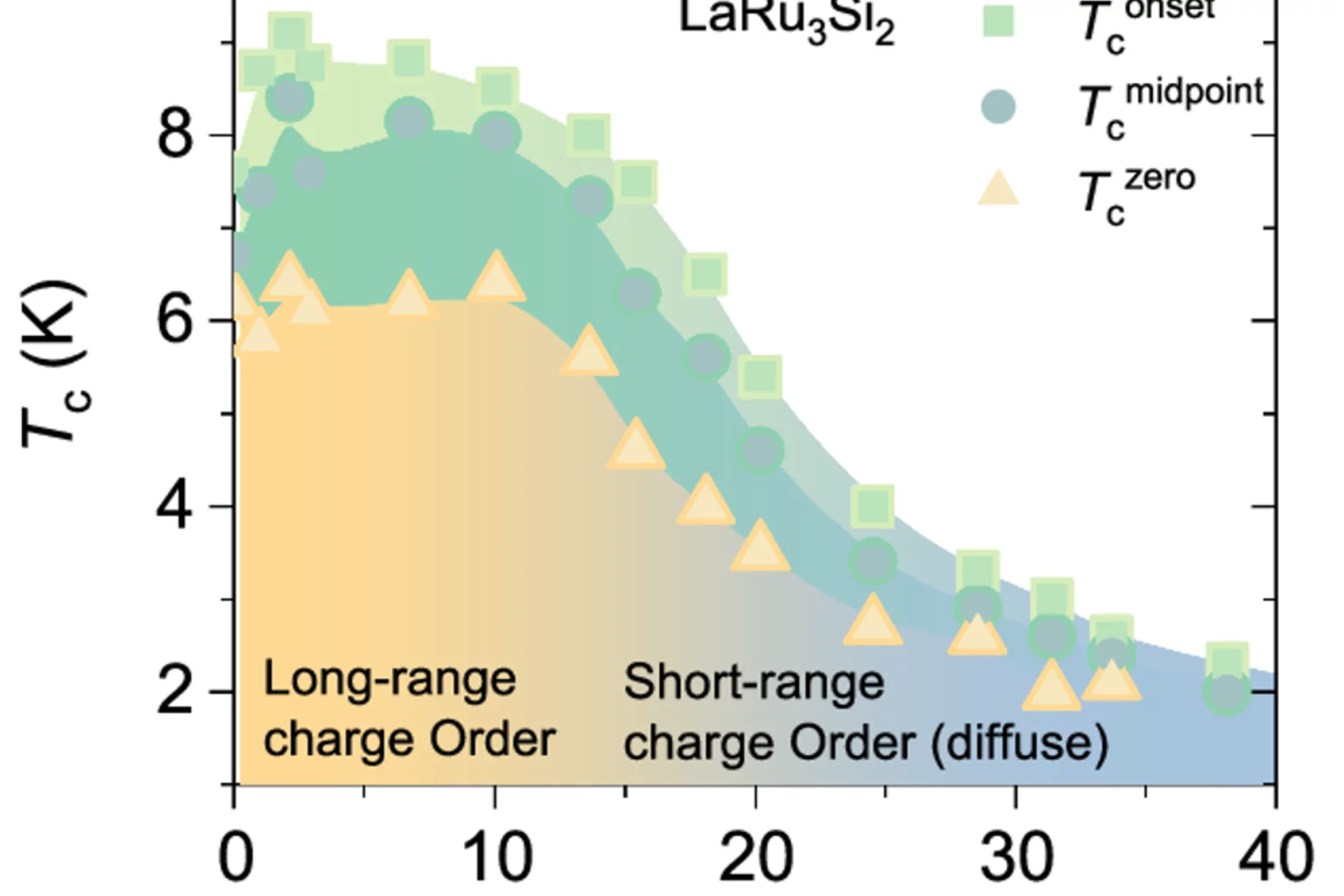
A New Quantum Landscape: Coexisting High-Tc Superconductivity, Magnetism, and Complex Charge Order in LaRu3Si2.
Despite intense research on kagome superconductors, many fundamental questions remain—especially regarding the unconventional nature of their charge order and superconducting phases. These materials are rich in complexity, and to truly unravel their behavior, a broad and integrated approach is essential. In our study ...
Cracking the Challenge of Steel–Copper Fusion
Why do cracks appear when joining steel and copper? We track the mechanisms in real time to find out.
Study reveals: Smoke from crop residue burning worsens air pollution in Indian cities
Identifying the main source of air pollution in Indian cities is crucial to reducing the many deaths caused by fine particulate matter (PM₂.₅) – deaths that during the harvest season can account for up to half of all air pollution-related fatalities. An international research team lead by the Paul Scherrer Institute (PSI), funded by the Swiss Agency for Development and Cooperation (SDC) has investigated in detail the sources of the organic components of fine particulate matter in the northern Indian cities of Delhi and Kanpur, located in the Indo-Gangetic Plain. Using novel high-resolution molecular measurement techniques and advanced data analysis, the researchers were able to precisely identify and quantify the sources of organic fine particulate matter.
Congratulations to Sonali for winning the ACM student research competition !
Congratulations to Sonali for winning the ACM student research competition that took place at PASC 2025 in Brugg as part of the SIGHPC ACM SIG conference
Transmutex at PSI Hotlab
Transmutex produces design and software for an accelerator-driven reactor able to transmute long-lived transuranic nuclides and fission products from the classic Uranium cycle for power production.
Benefits such as Nuclear waste volume reductions above a factor of 6 and HAW lifetime reductions to << 1000 years will be attained.
Breeding U-233 in Th fuel in the reactor integrates Transmutex’s design with existing commercial PHWR and LWR in providing fuel and fissionable material for their continued operation.
At PSI hotlab, Transmutex develops the manufacturing of porous metal pellets aiming to produce experimental Th pellets for further investigation utilizing PSI’s and hotlabs’ research capabilities. The new design will unify the advantages of metallic fuel such as high fissile density, ease of fabrication, and more while mitigating classic issues of compact metallic fuel related to the buildup of fission gas.
Successfully produced inactive analogues are illustrating the prove of concept of the design.
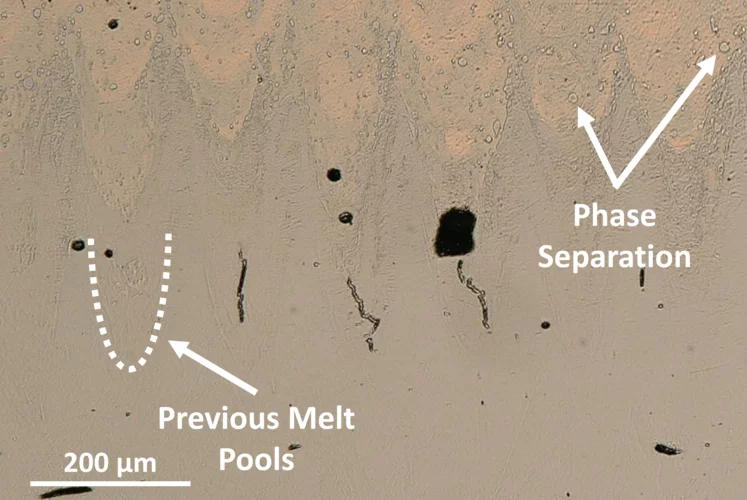
Interfacial Phase Formation in 316L–CuCrZr Hybrids
In-situ X-ray diffraction reveals how phase separation and fluid flow shape microstructure in laser-welded multi-material metal builds.
Sarbajit Banerjee wins Royal Society of Chemistry’s Centenary Prize
ETH Zürich and the Paul Scherrer Institut PSI scientist Professor Sarbajit Banerjee has been named winner of the Royal Society of Chemistry’s Centenary Prize in recognition of brilliance in research and innovation.
Phase by Phase: How Stainless Steel and IN625 Solidify Together
Where steel meets superalloy: real-time X-ray snapshots reveal how composition and cooling shape metal during 3D printing
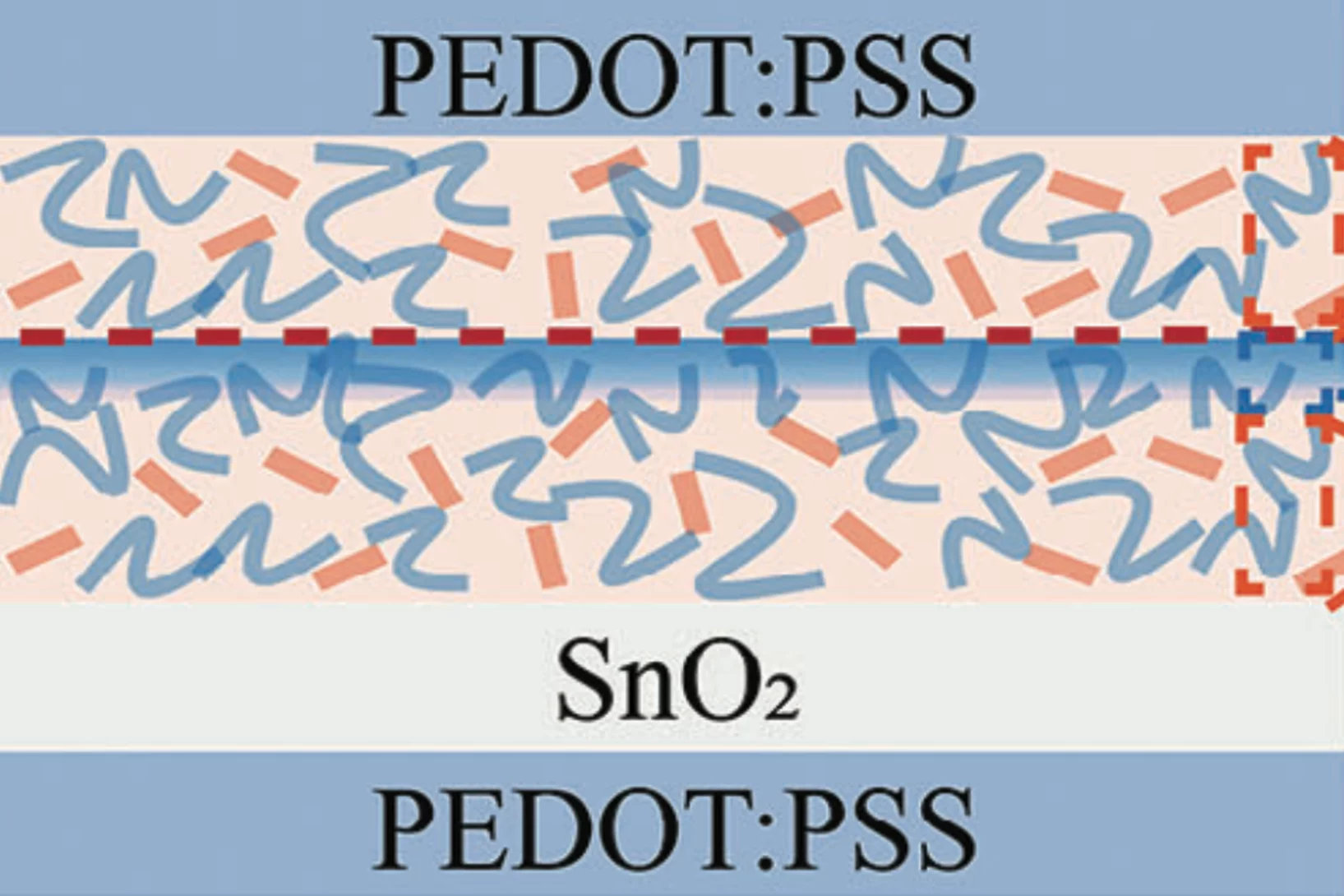
Understanding and Addressing the Performance Asymmetry Issue in Semitransparent Laminated Organic Photovoltaic Devices
Organic photovoltaics (OPVs) offer a promising solution for indoor energy harvesting. However, fundamental investigations to understand and optimize industrial processes such as roll-to-roll lamination for upscaling remain limited. This study investigates a critical failure mode in the upscaling of OPVs.
One major challenge ...
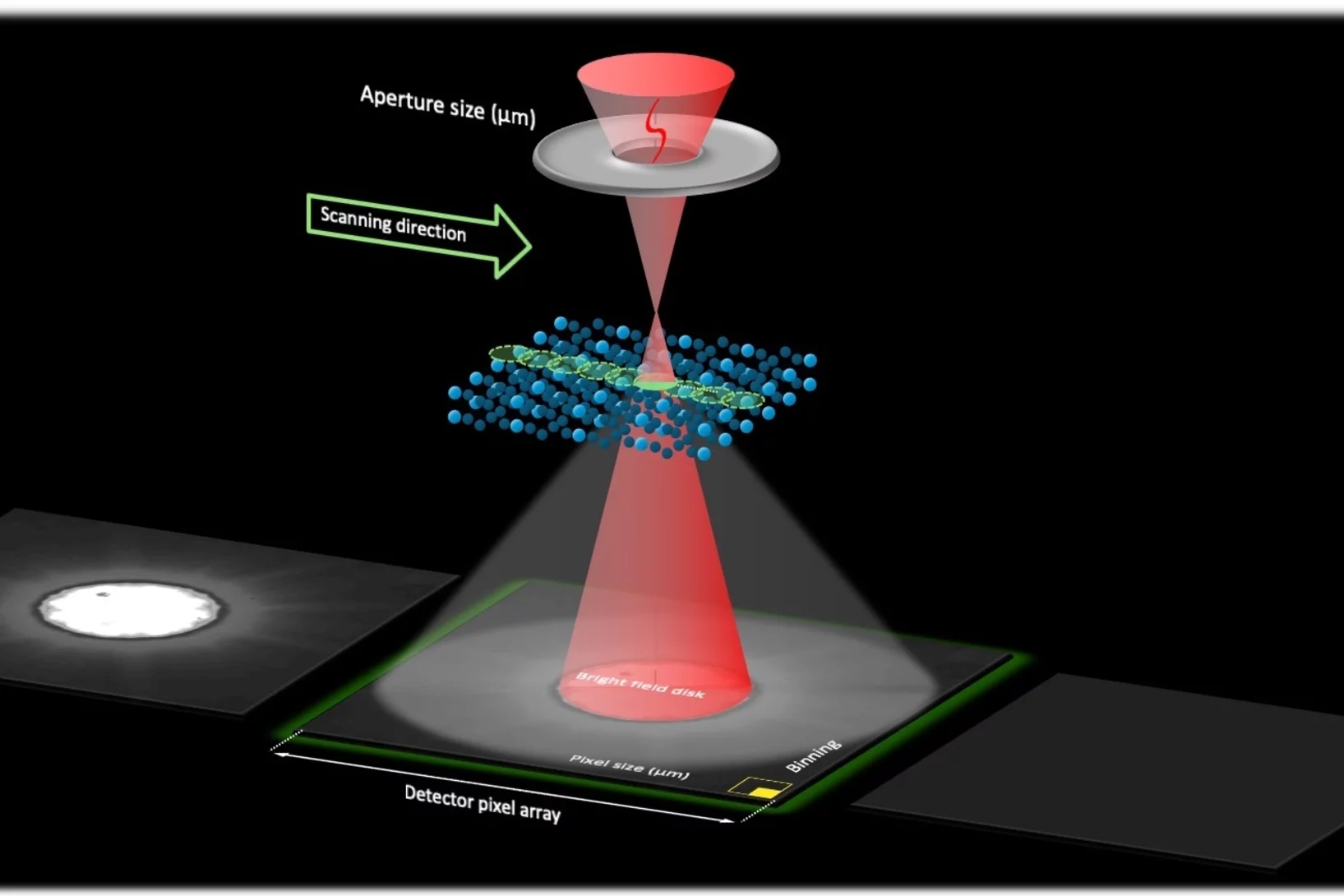
Gold nanoparticle dynamics on graphene probed by convergent beam electron diffraction.
Dynamics of single Au nanoparticles on graphene were probed simultaneously in real- and diffraction space by time-series convergent beam electron diffraction.
Absence of Altermagnetic Magnon Band Splitting in MnF2
Altermagnets are collinear compensated magnets in which the magnetic sublattices are related by rotation rather than translation or inversion. One of the quintessential properties of altermagnets is the presence of split chiral magnon modes. Recently, such modes have been predicted in MnF2.
Here, we report inelastic neutron scattering results ...
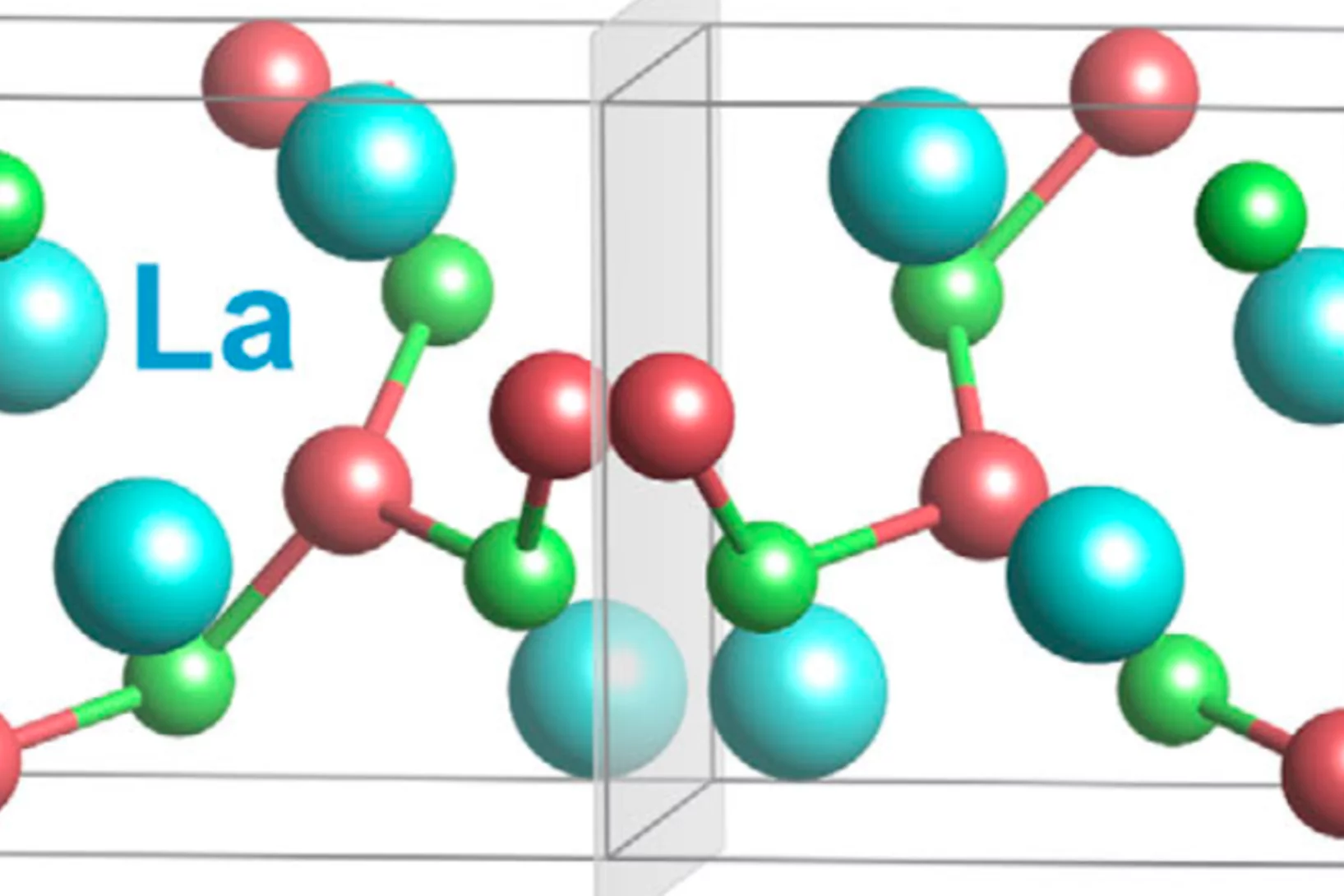
Pressure effect on the spin density wave transition in La2PrNi2O6.96
High-pressure studies reveal a stark contrast between the superconducting properties of double-layer Ruddlesden-Popper (RP) nickelates La2PrNi2O7 and La3Ni2O7. While La2PrNi2O7 exhibits bulk superconductivity, La3Ni2O7 displays filamentary behavior, suggesting that superconductivity is confined to phase interfaces rather than the bulk. Since magnetism emerges ...
Research Software Engineering (RSE) Movement at PSI
The online information event on the “Research Software Engineering (RSE) Movement” at PSI on Wednesday 7th May, 2025 was a quite success with an unexpected high number of 100+ participants!
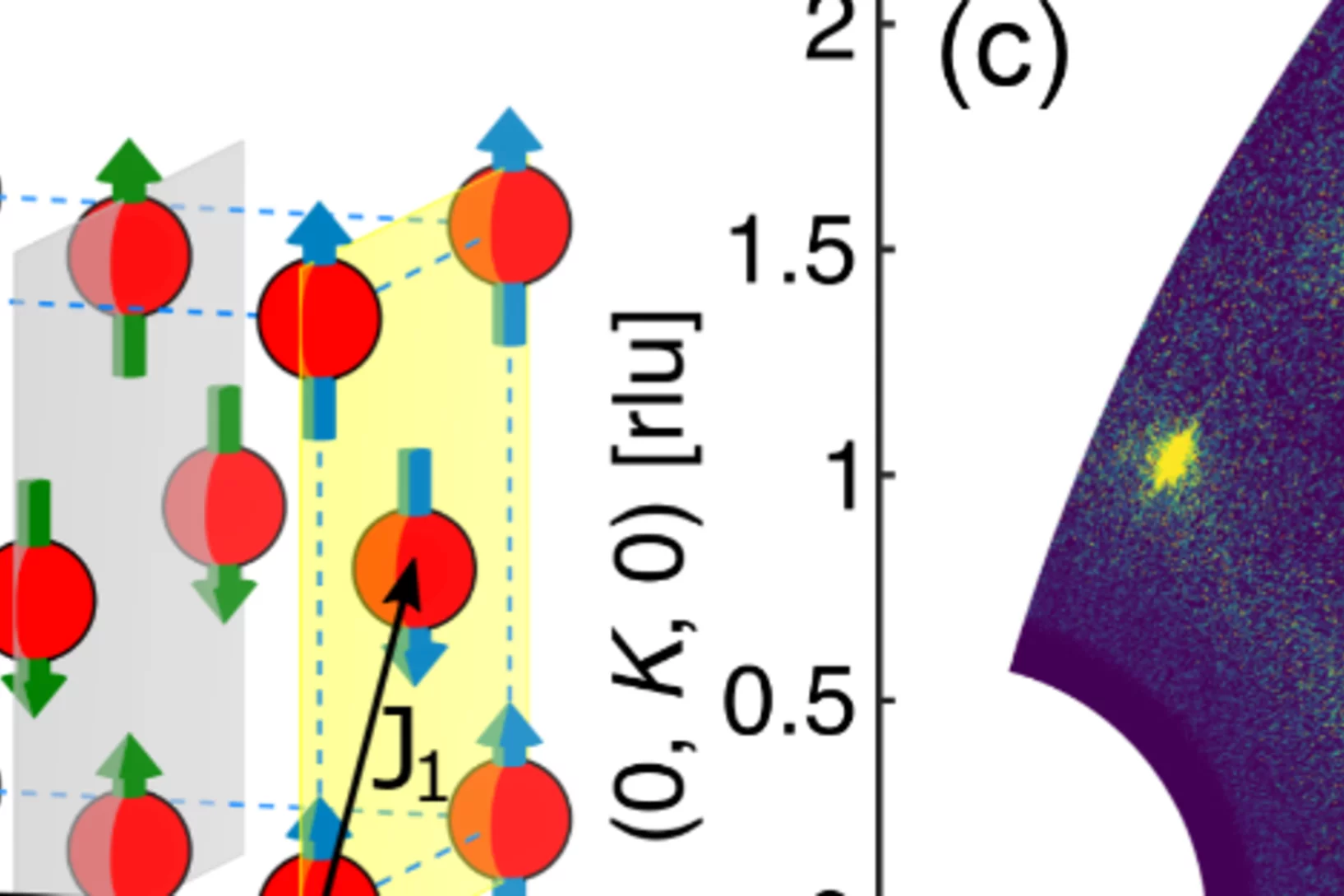
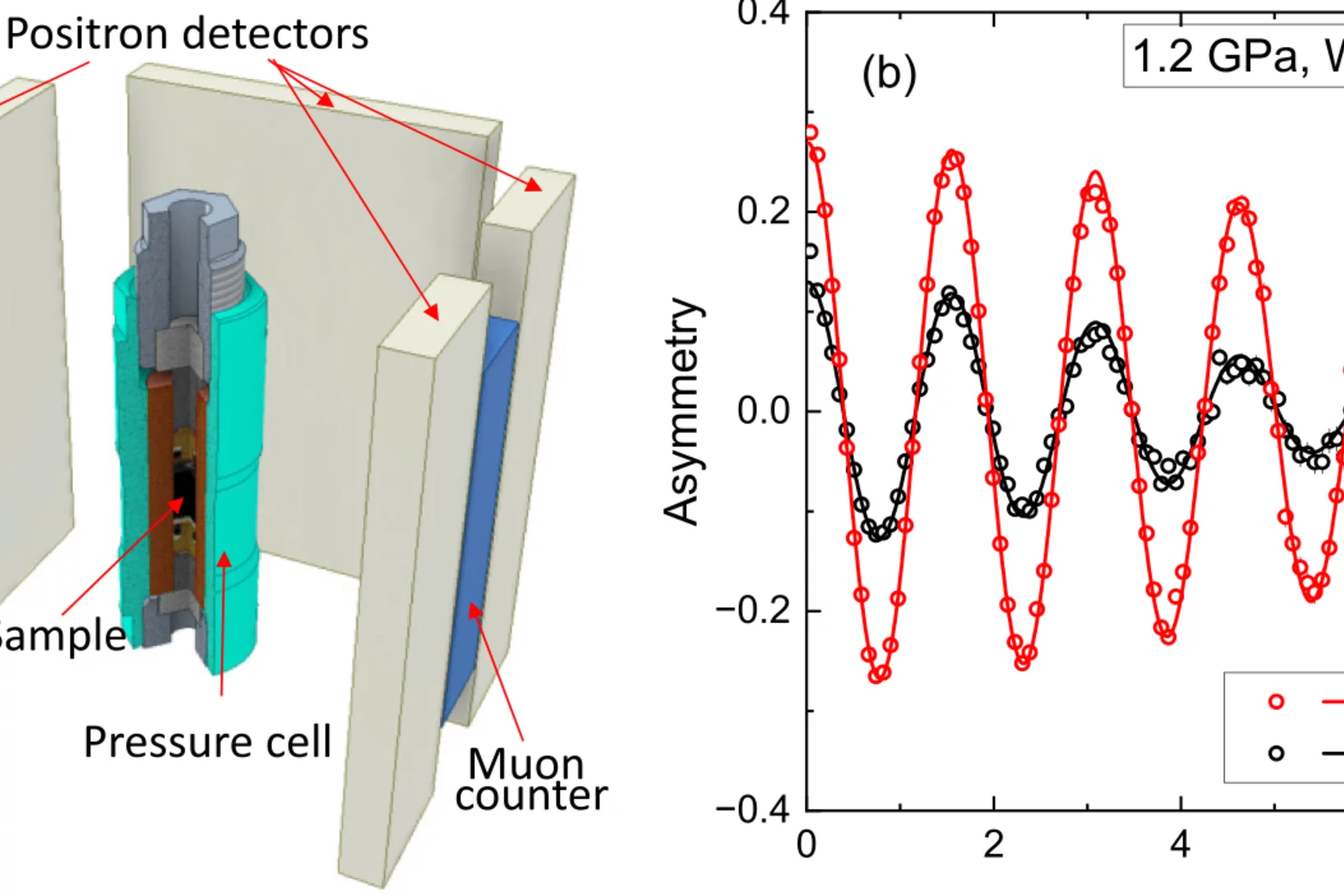
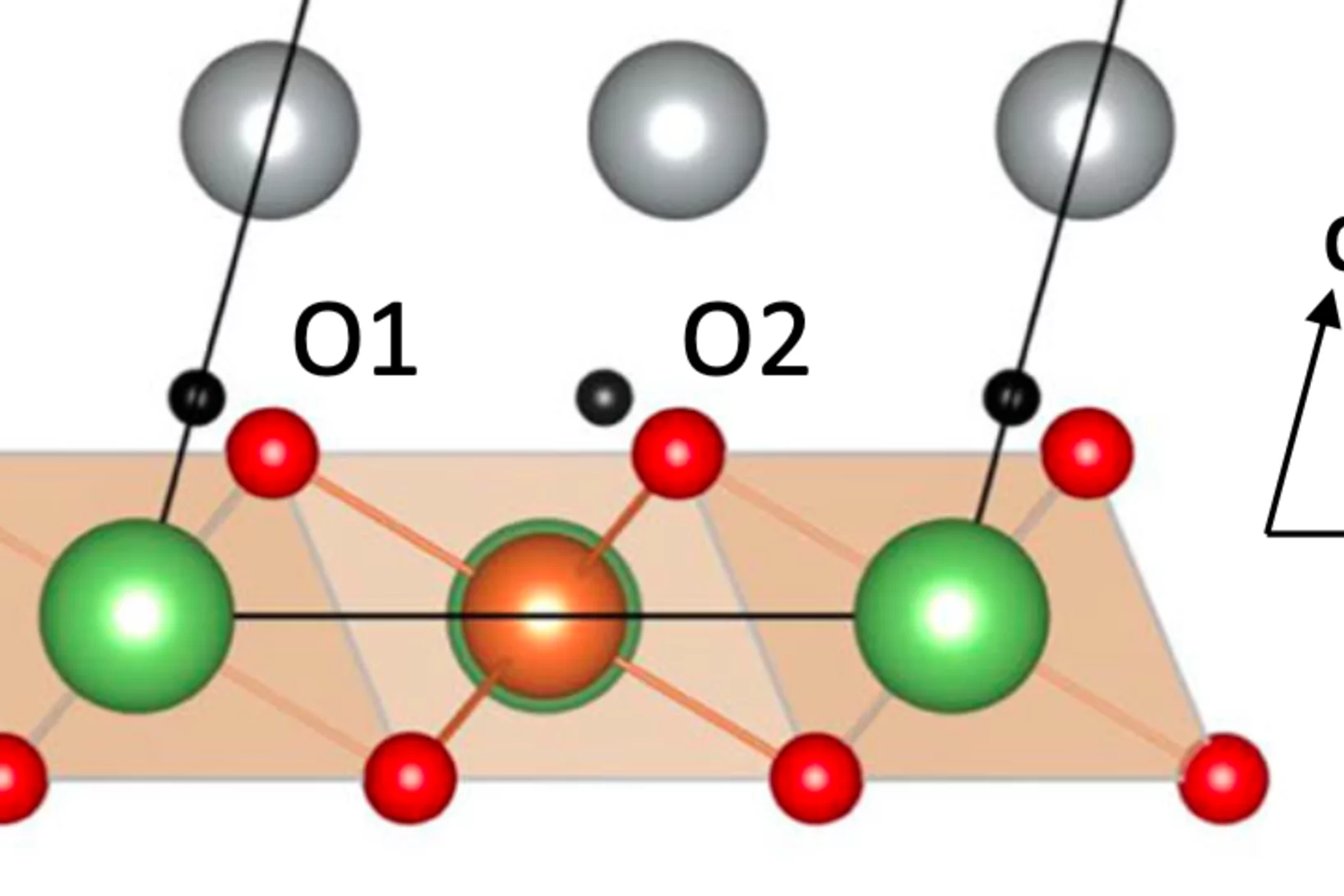
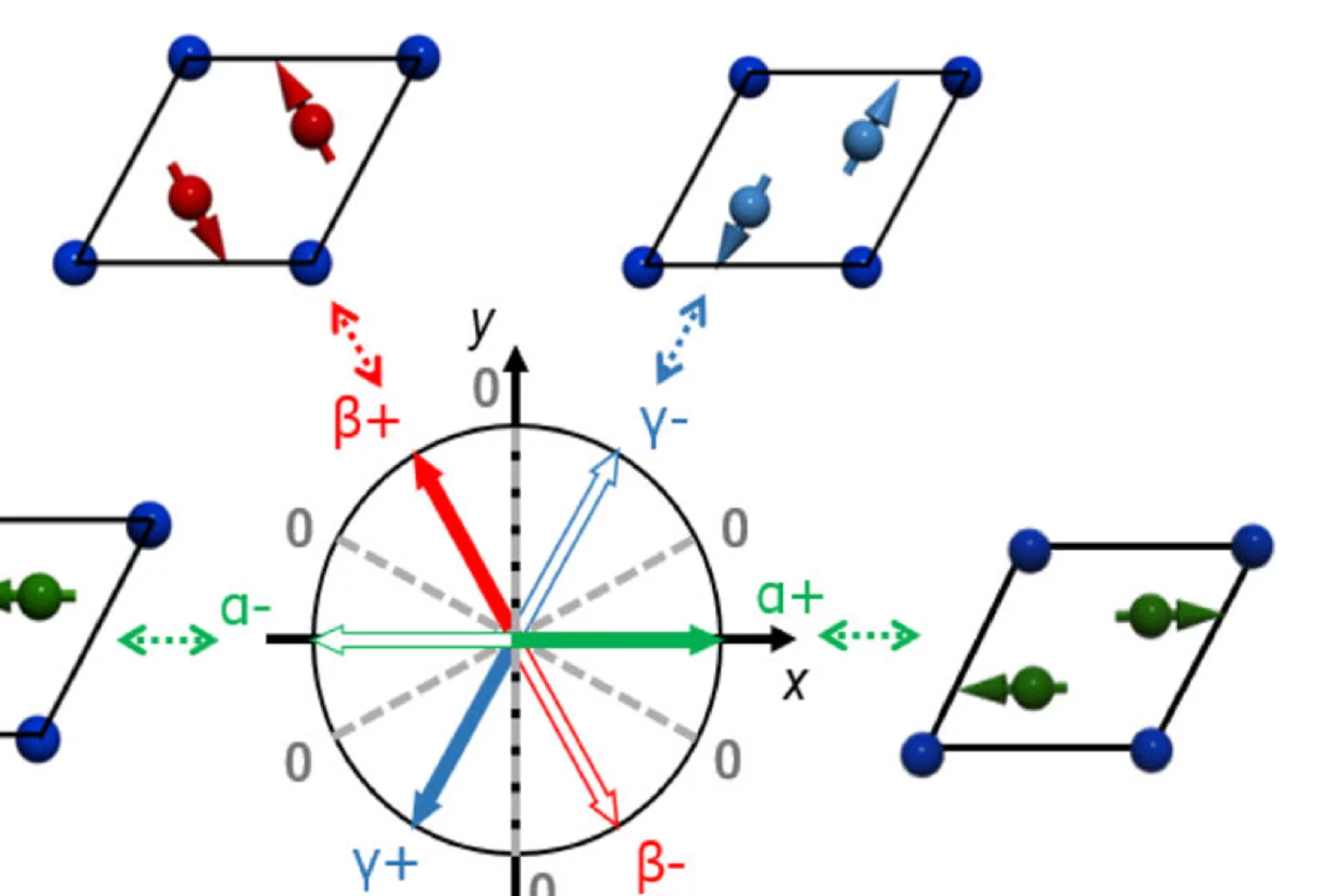
Pressure tuning of competing interactions on a honeycomb lattice
Exchange interactions are mediated via orbital overlaps across chemical bonds. Thus, modifying the bond angles by physical pressure or strain can tune the relative strength of competing interactions. Here we present a remarkable case of such tuning between the Heisenberg (J) and Kitaev (K) exchange, which respectively establish magnetically ordered and spin liquid phases on a honeycomb lattice. We observe ...
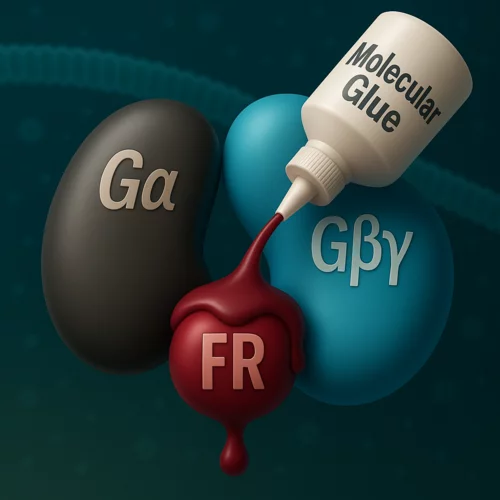
From coral berries to new therapies: uncovering the molecular glue mechanism of natural compounds
Researchers at the Center for Life Sciences and the Center for Scientific Computing, Theory, and Data at the Paul Scherrer Institute have identified the mechanism by which certain natural compounds interfere with cellular signaling. These ‘molecular glues’ have a therapeutic potential for the treatment of specific cancer types. Their latest study on this topic has been published in the journal Proceedings of the National Academy of Sciences of the United States of America.
Dr. Yingfang He has been honored with the Alavi-Mandell Award 2025
We congratulate Dr. Yingfang He for the excellent research work she did during her time at the Center for Radiopharmaceutical Sciences.
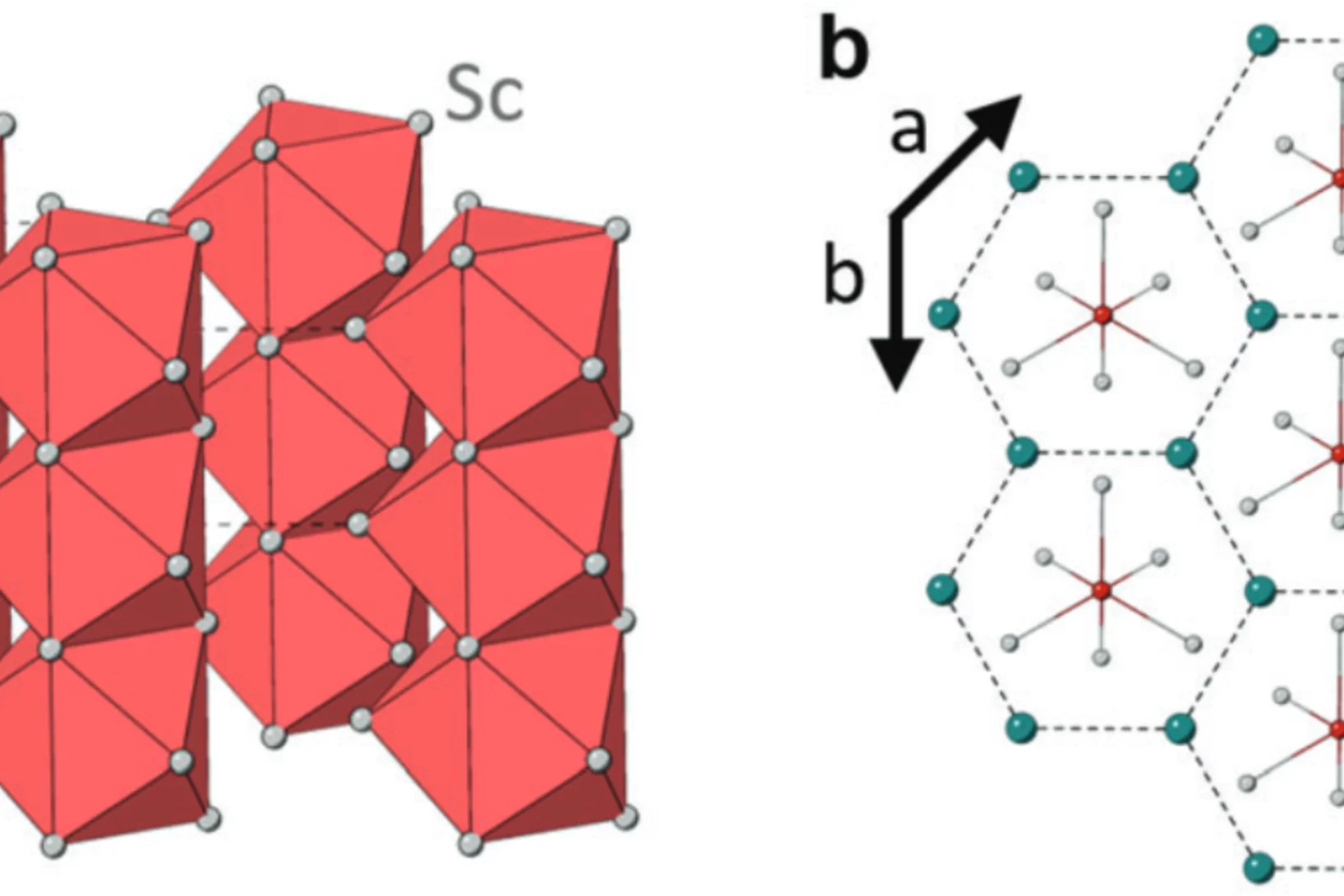
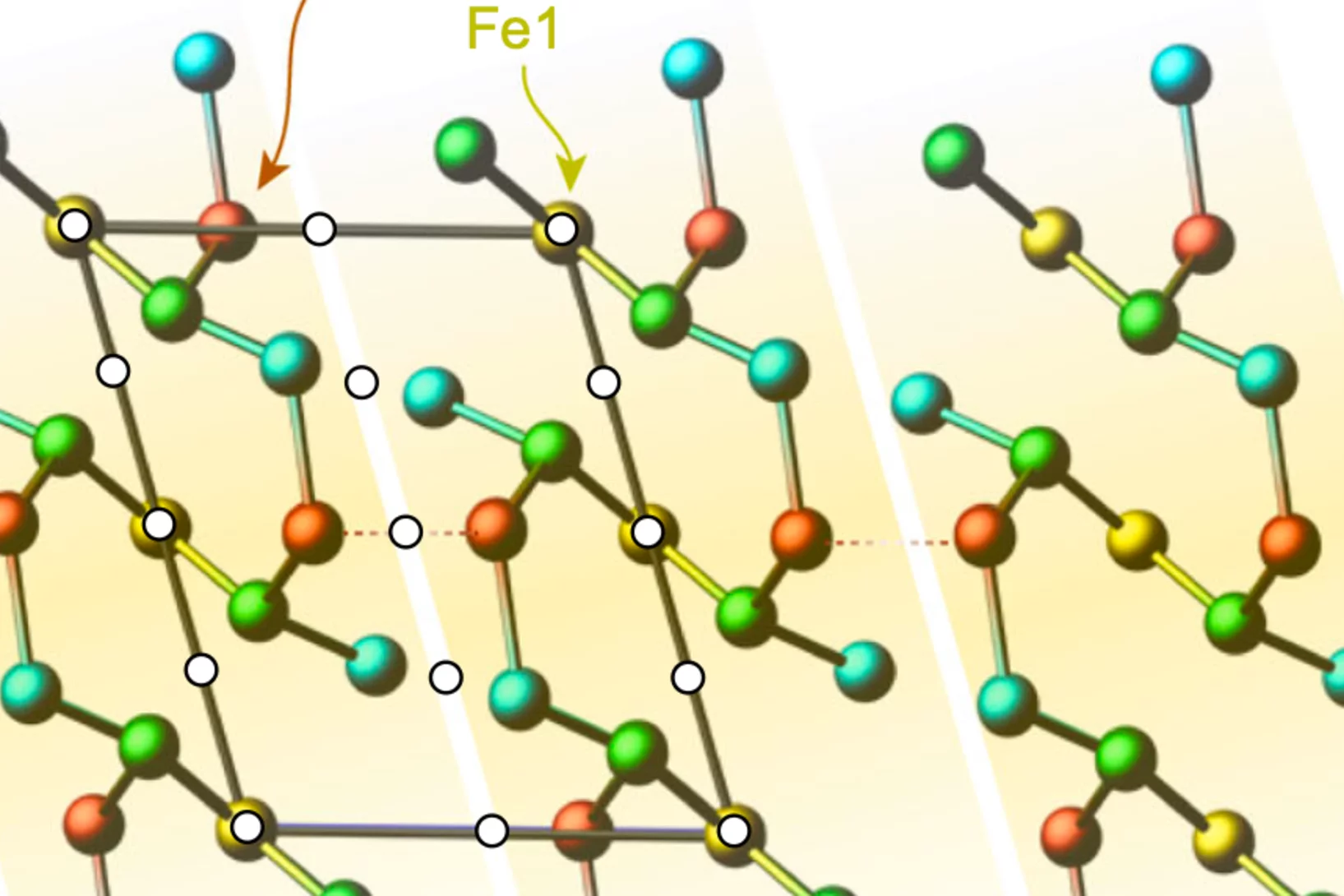
Tailoring the Normal and Superconducting State Properties of Ternary Scandium Tellurides, Sc6MTe2 (M = Fe, Ru, and Ir) Through Chemical Substitution
The pursuit of a unifying theory for non-BCS superconductivity has faced significant challenges. One approach to overcome such challenges is to perform systematic investigations into superconductors containing d-electron metals in order to elucidate their underlying mechanisms. Recently, the Sc6MTe2 (M = d-electron metal) family has emerged as a unique series of isostructural compounds exhibiting superconductivity across a range of 3d, 4d, and 5d electron systems.
In this study, muon spin rotation, neutron diffraction, and magnetization techniques are employed to probe ...
Achieving Uniform Phase Structure for Layer-by-Layer Processed Binary Organic Solar Cells with 20.2% Efficiency
Layer-by-layer (LBL) deposition has become a facile and promising method to fabricate highly efficient organic solar cells (OSCs). However, characterization and optimization of 3D morphology remain a grand challenge for LBL- processed active layers, and their correlation with photovoltaic properties of OSC devices is not clear to date.
Here, to address this issue, ...
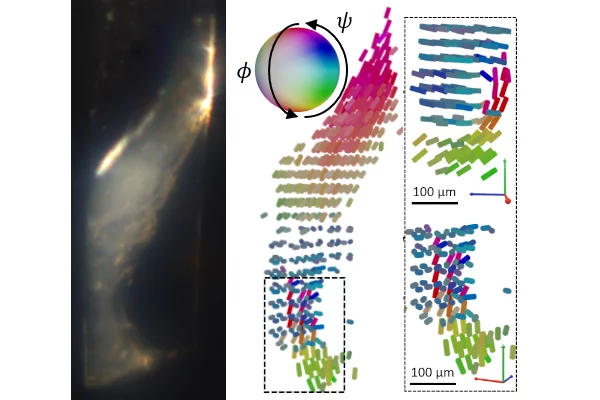
Nanostructure orientation in 3D with visible light by Tomographic Müller-Polarimetric Microscopy
We developed a new method, tomographic Müller-polarimetric microscopy (TMPM), that allows to retrieve at three-dimensional microscopic resolution the nanoscale structural information of the ultrastructure probed with polarized light in a non-destructive manner using a low cost and experimentally simple optical setup.
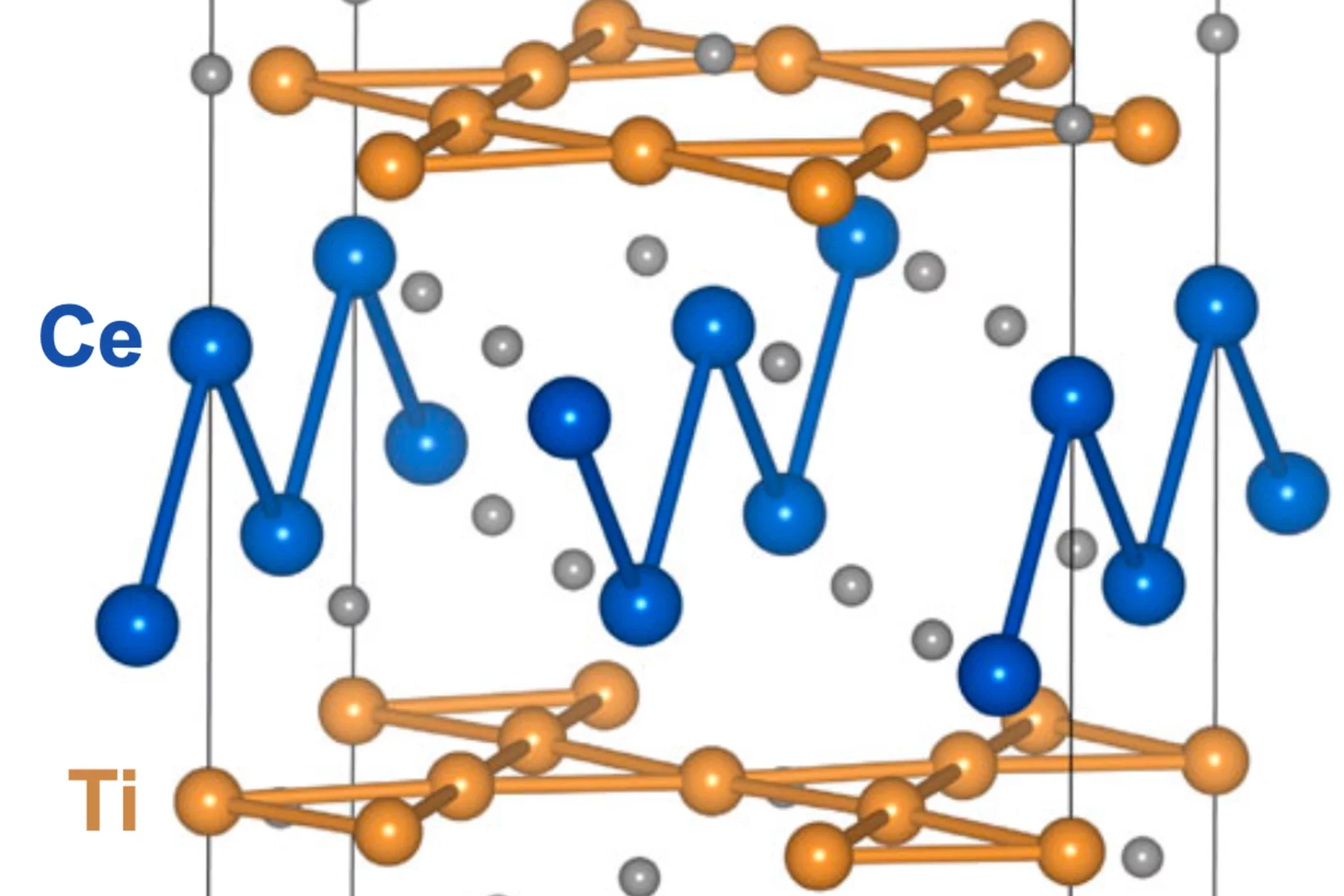
Spin density wave and van Hove singularity in the kagome metal CeTi3Bi4
Kagome metals with van Hove singularities near the Fermi level can host intriguing quantum phenomena such as chiral loop currents, electronic nematicity, and unconventional superconductivity. However, to our best knowledge, unconventional magnetic states driven by van Hove singularities–like spin-density waves–have not been observed experimentally in kagome metals. Here, we report ...
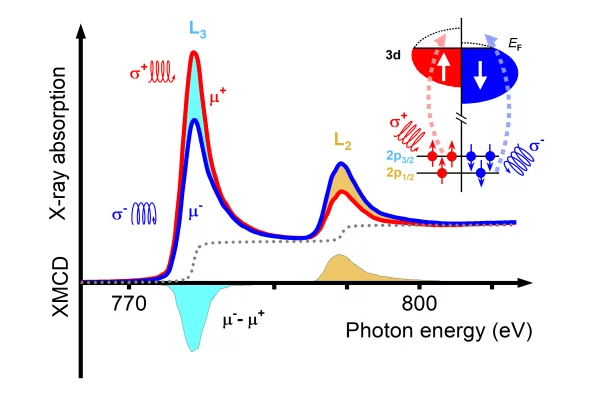
Primer on X-ray magnetic circular dichroism
X-ray magnetic circular dichroism (XMCD) is a magneto-optical effect that describes the difference in absorption between left and right circularly polarized X-rays by a magnetized material. It has been widely applied to the study of magnetic systems and of magnetic phenomena and its unique capabilities make it a fundamental tool for the study of novel magnetic phenomena and new materials systems.
Advancing Biogas Quality: Tackling Siloxane Challenges for Smooth Energy Transition
Siloxanes, present in everyday items, can compromise the efficiency and durability of bioenergy systems, even at trace levels. Monitoring and quantifying these impurities are critical for improving biogas quality and expanding its role in renewable energy. However, sampling biogas and storing samples containing siloxanes for analysis remain a significant challenge.
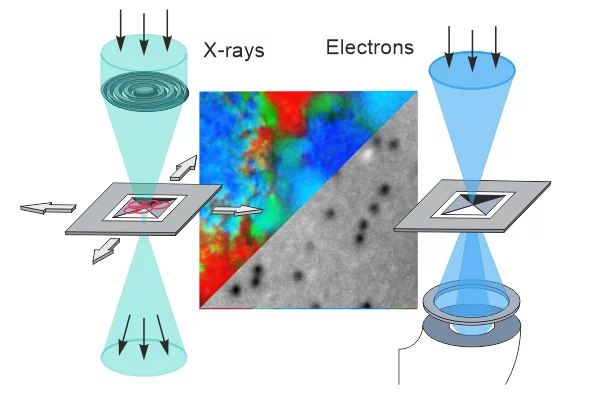
Correlating transmission electron and soft x-ray microscopy to bridge atomic- and mesoscales
Transmission electron and soft x-ray microscopy have contributed significantly to our understanding of phenomena in fields ranging from biology to materials science. In this review, we present recent developments in combining transmission electron and soft x-ray microscopy techniques, including progress in sample environment, and in situ and operando approaches and highlight the unique opportunities offered by fully correlative transmission electron and soft x-ray microscopy.
4D imaging of frost heave and ice lens growth in silt using neutron and x-ray computed tomography
There are substantial changes in soil structure in regions where the soil is freezing. Water movements in the freezing soil introduce level changes beyond what can be expected by the expansion of water when it freezes. The impact of frost heave is seasonal damage to our built environment ...
Antiferrodistortive and ferroeletric phase transitions in freestanding films of SrTiO3
Epitaxially grown thin films are commonly used to strain engineer electronic properties by the choice of a substrate, and therefore do not match bulk properties (leading to properties that deviate from the bulk material). Free standing ultrathin oxide films are expected to preserve the bulk-like properties due to the absence of substrate influence. However, we show that this expectation is not fulfilled with ultrathin free standing SrTiO3, as they get ferroelectric at 80K.
Momentum-resolved fingerprint of Mottness in layer-dimerized Nb3Br8
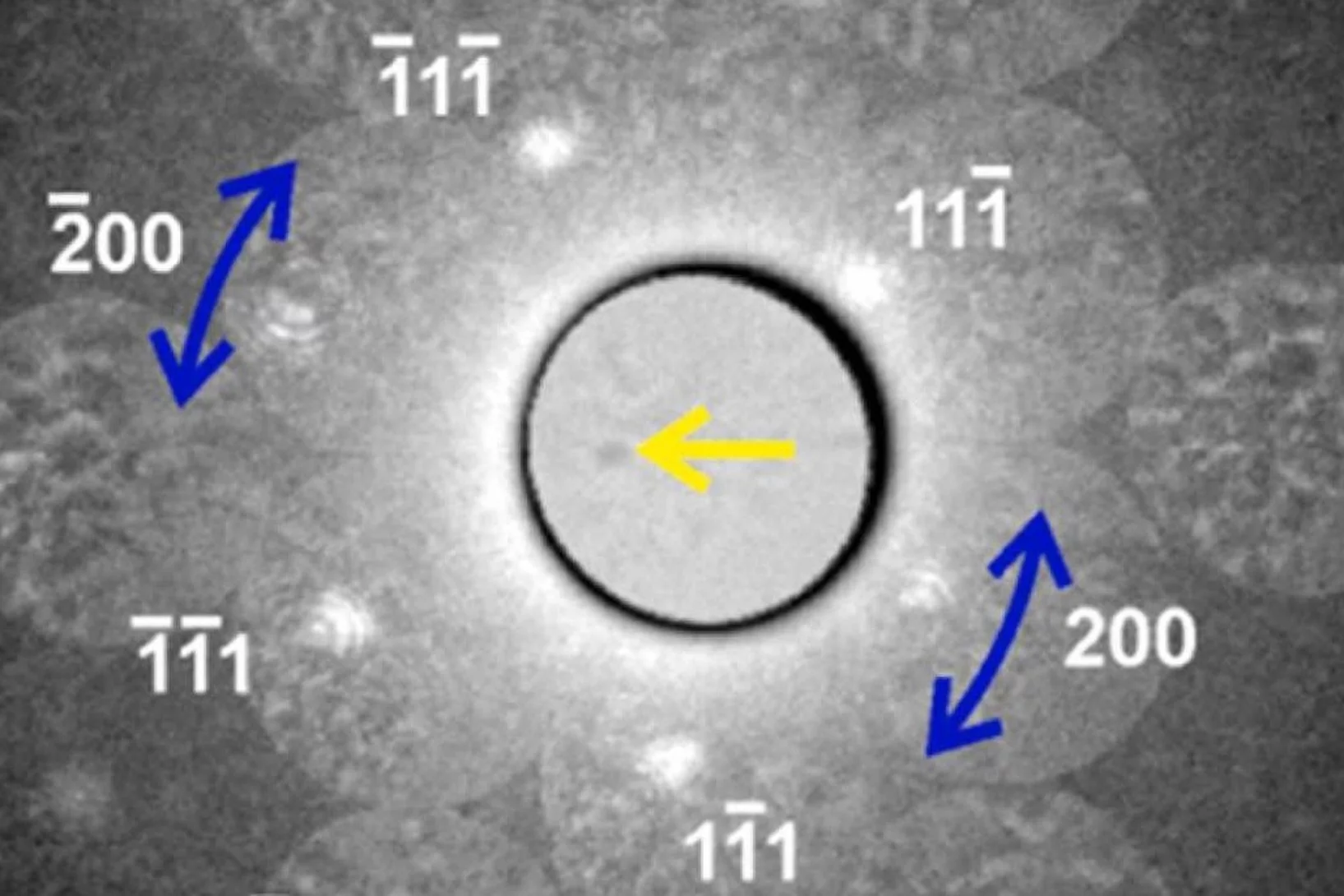
Crystalline solids can become band insulators due to fully lled bands, or Mott insulators due to strong electronic correlations. While Mott insulators can theoretically occur in systems with an even number of electrons per unit cell, distinguishing them from band insulators experimentally has remained a longstanding challenge.
In this work, we present ...
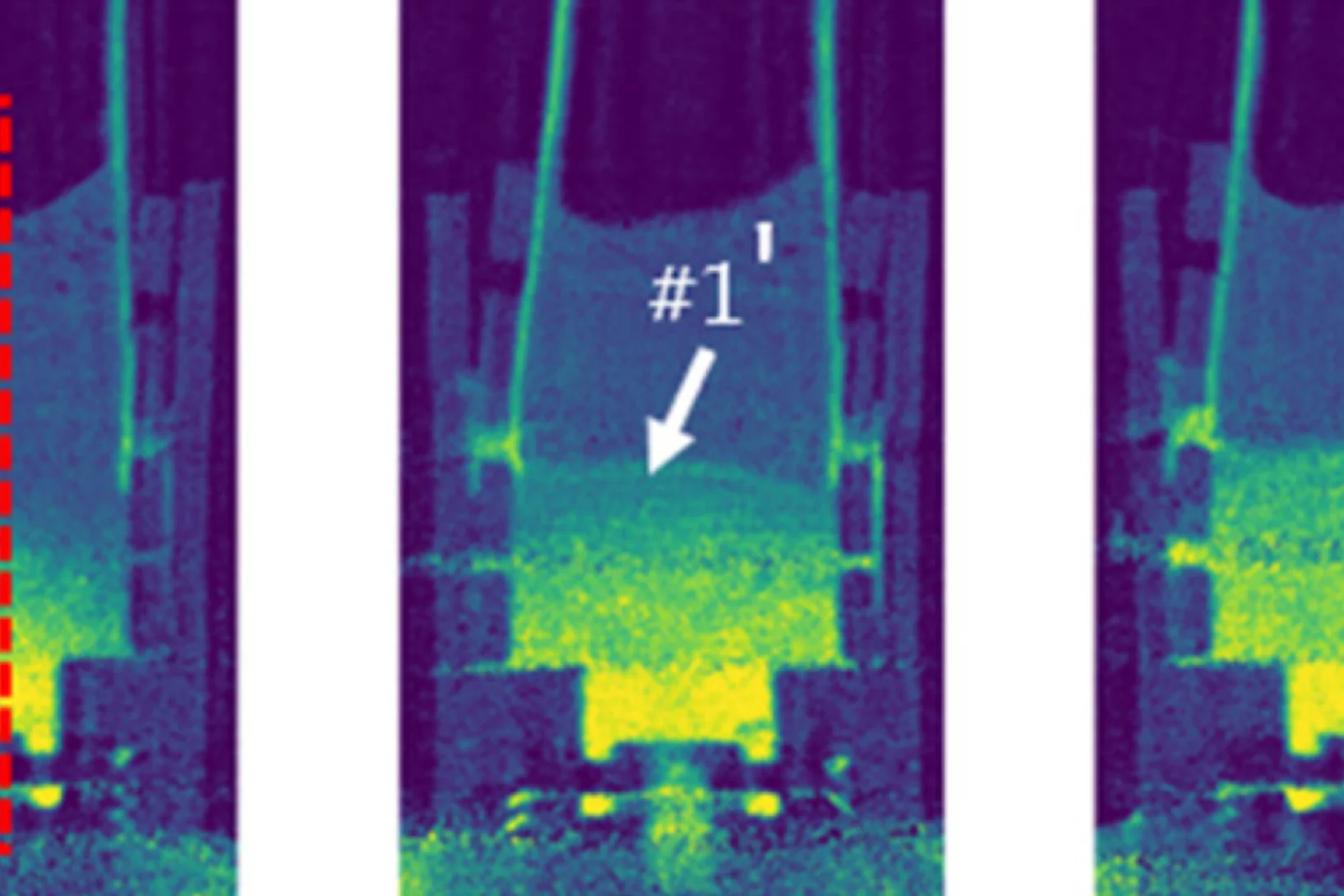
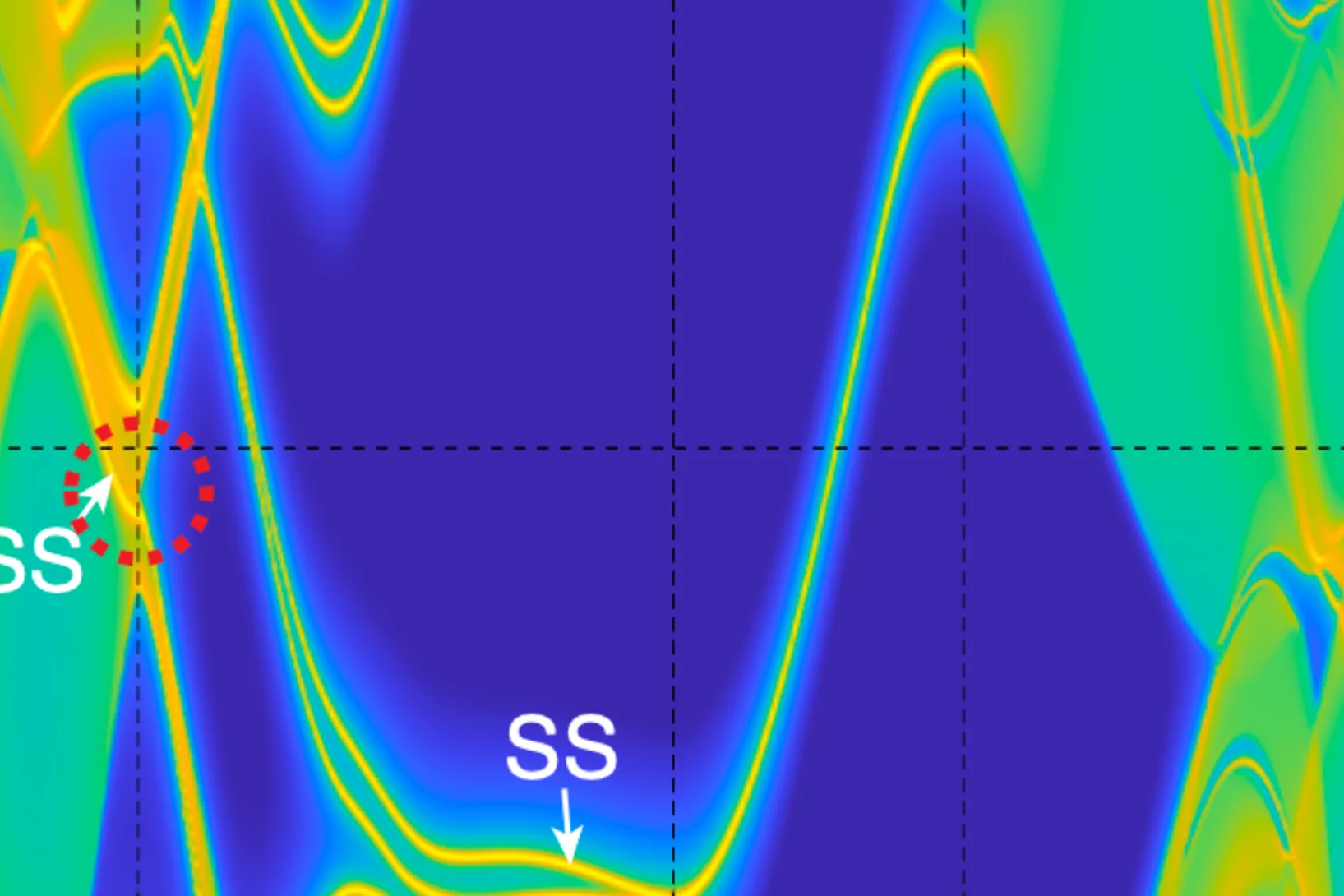
Superconductivity and a van Hove singularity confined to the surface of a topological semimetal
The interplay between topology and superconductivity generated great interest in condensed matter physics. Here, we unveil an unconventional two-dimensional superconducting state in the Dirac nodal line semimetal ZrAs2 which is exclusively con ned to the top and bottom surfaces within the crystal’s ab plane.
As a remarkable consequence ...
Emergence of topological Hall effect from a fluctuation-based dynamic origin
The topological nature of the electronic bands or spin structure has direct manifestation in experimentally measured Hall conductivity. The extra topological (or geometrical) component to the Hall effect (THE) usually emerges due to multi-k structures, which inherently possess a finite static scalar spin chirality (SSC). Generating a THE in a single-k structure necessitates the consideration of the dynamical origin of SSC, the real material examples of such cases remain scarce to date.
Water gets in shape for VUV absorption
Nanometre‑thin, free‑flowing liquid sheets now let Swiss Light Source users record pristine VUV absorption spectra of water, and soon any solvent.
Zero-field Hall effect emerging from a non-Fermi liquid in a collinear antiferromagnet V1/3NbS2
Magnetically intercalated transition metal dichalcogenides (TMDs) provide a versatile three-dimensional (3D) material platform to explore quantum phenomena and functionalities that emerge from an intricate interplay among magnetism, band structure, and electronic correlations.
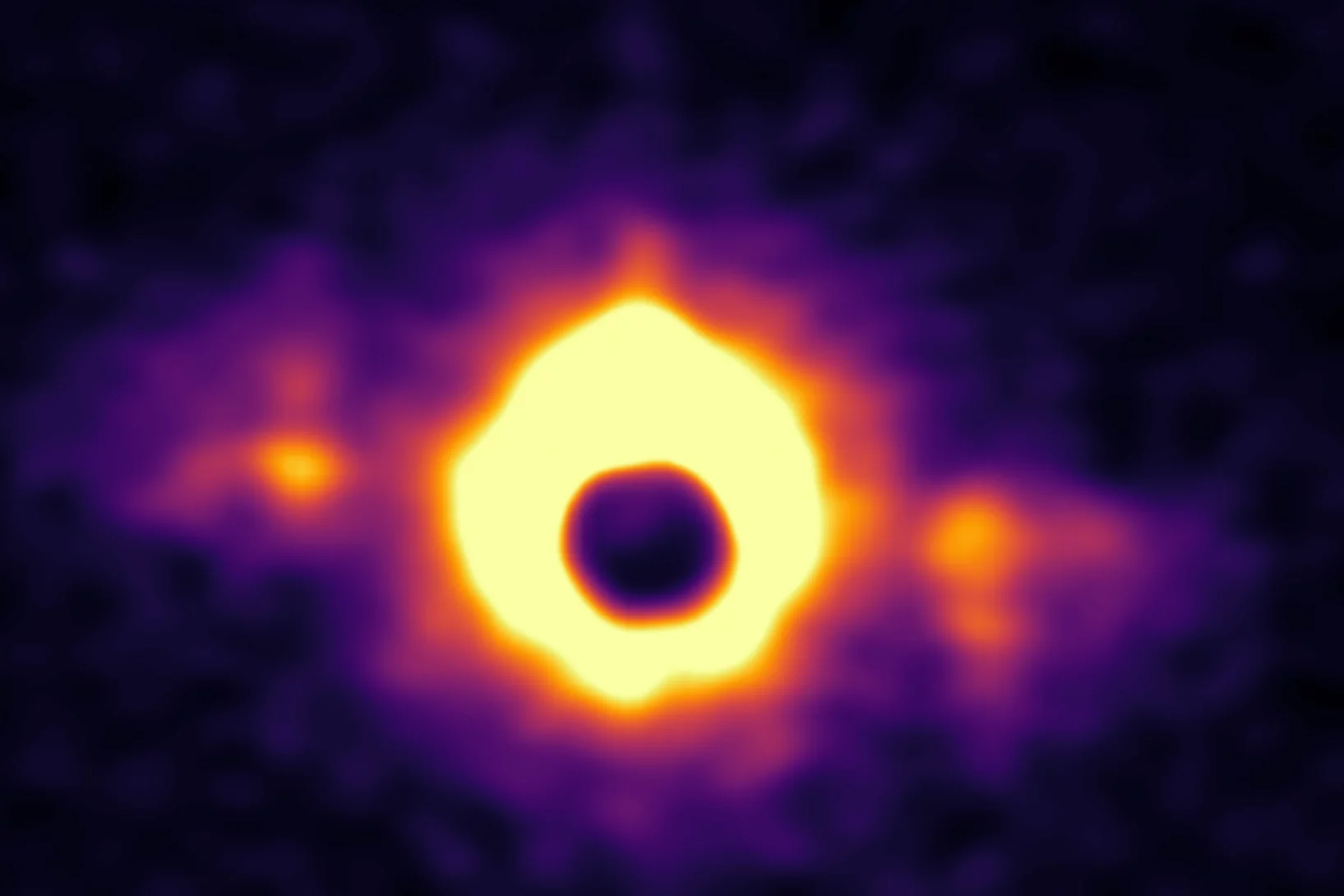
Generation of Neutron Airy Beams
The Airy wave packet is a solution to the potential-free Schrödinger equation that exhibits remarkable properties such as self-acceleration, nondiffraction, and self-healing. Although Airy beams are now routinely realized ....