Researchers at the Center for Life Sciences and the Center for Scientific Computing, Theory, and Data at the Paul Scherrer Institute have identified the mechanism by which certain natural compounds interfere with cellular signaling. These ‘molecular glues’ have a therapeutic potential for the treatment of specific cancer types. Their latest study on this topic has been published in the journal Proceedings of the National Academy of Sciences of the United States of America.
New medicines can be found in unexpected places
FR900359 (FR) and YM-254890 (YM) are natural compounds that have become important tools in medical research. FR comes from the ornamental plant Ardisia crenata (coral berry), where it's produced by bacteria living in the plant's leaves. YM is made by soil bacteria called Chromobacterium. These compounds are special because they can block specific communication pathways in cells by inhibiting proteins called Gq and G11, which act as molecular switches. Scientists now use FR and YM to understand how cells communicate [1, 2] and how these communication pathways may be involved in human disease. This research has revealed promising applications for treating eye cancer (uveal melanoma), where faulty Gq/G11 proteins drive tumor growth. Laboratory studies show that blocking these abnormal proteins with FR can stop cancer cell growth and trigger their self-destruction, pointing to potential new treatments for this difficult-to-treat cancer [3]. To transform these promising compounds into effective medicines, however, researchers needed to understand how they work at the fundamental level.
Understanding how medicines work at the atomic level
Heterotrimeric G proteins, such as Gq and G11, function as crucial molecular switches in cellular communication. To build on the medical potential of FR and YM, researchers face a key challenge: understanding exactly how these compounds block these molecular switches. This knowledge is essential for transforming these natural compounds into effective medicines with improved properties such as better absorption, stability, or targeted delivery. "To develop better drugs based on FR and YM, one needs to see precisely how these molecules interact with their target proteins", explains Dr. Deupi (CSD/CLS), one of the study's lead researchers. "It's like trying to improve a key—you need to know the exact shape of the lock. But in biology, both the key –the small molecule– and the lock –the protein– are dynamic and constantly slightly reshaping themselves”. To capture these intricate molecular interactions, the team turned to the Swiss Light Source (SLS). Using crystallography techniques, they were able to capture high-resolution 3D images of the G protein bound to both FR and YM. These structures, at unprecedented resolution, revealed surprising insights. "What we discovered was remarkable", says Jonas Mühle (CLS), the first author of the study. "These compounds don't just block one part of the protein as previously thought—they actually act as 'molecular glues' that hold multiple parts of the signaling machinery together, preventing it from activating". This unexpected dual mechanism explains why these compounds are so effective at shutting down G protein signaling completely. The detailed structures obtained at SLS provide a precise blueprint that medicinal chemists can now use to design improved versions of these compounds, potentially leading to new treatments for diseases driven by faulty Gq/11 proteins.
The science
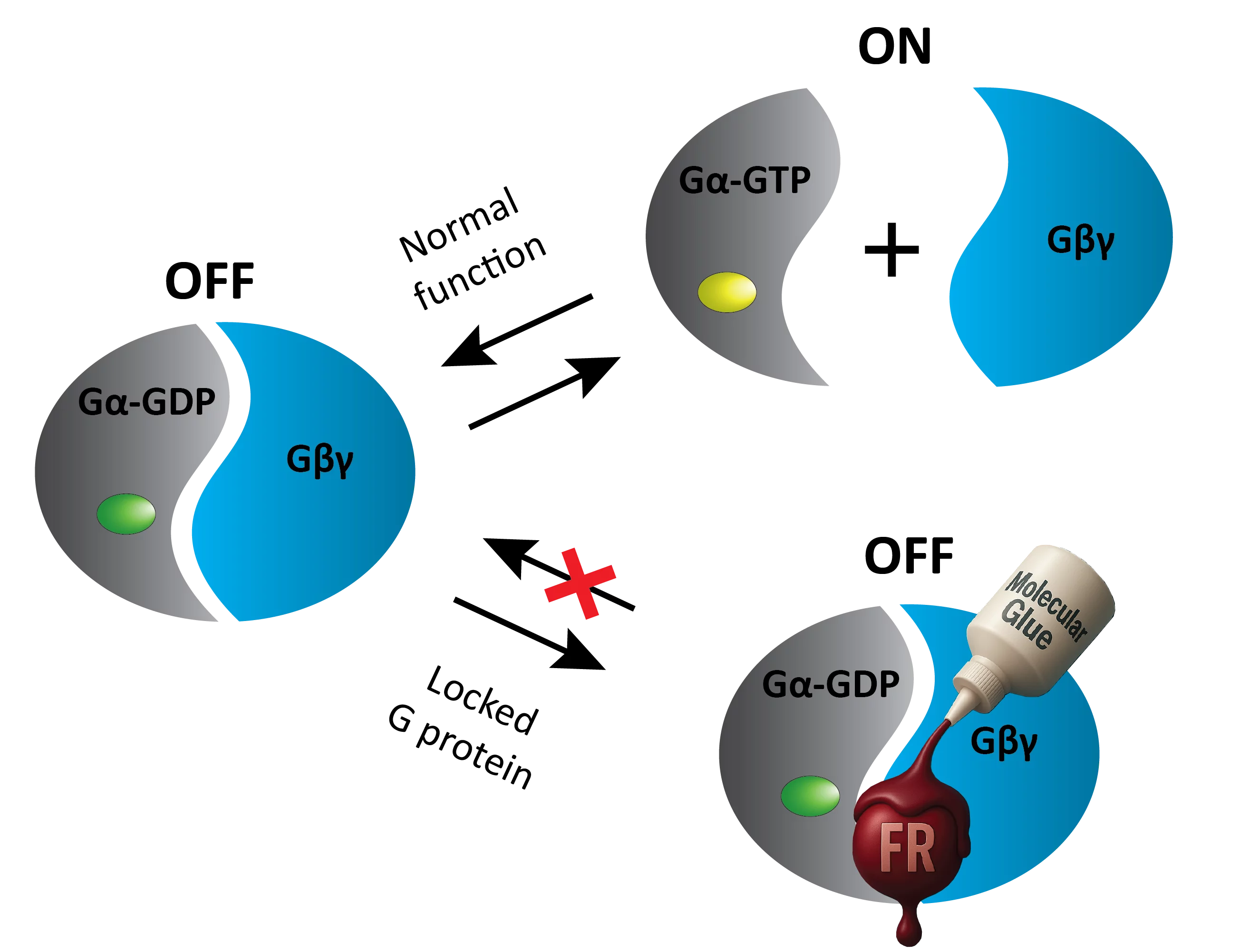
In healthy cells, heterotrimeric G proteins alternate between an OFF-state (when the Gα subunit is bound to GDP and forms a heterotrimer with the Gβγ subunit) and an ON-state (when Gα is bound to GTP and separated from Gβγ). For researchers studying cellular communication, pharmacological tools to securely prevent this OFF-ON transition are crucial for investigating this molecular switch, and FR and YM are precisely such tools.
The prevailing scientific understanding had attributed their efficient and long-lasting inhibition of Gq/11 signaling solely to a wedge-like binding to the Gα subunit, preventing GDP release. However, the team’s research revealed a more complex mechanism. By using X-ray crystallography, biochemical and signaling assays, and cellular biosensors, the team showed that FR and YM also function as stabilizers of the Gα:Gβγ subunit interface. The remarkable high-resolution structures reveal a network of residues in Gα and two highly conserved amino acids in Gβ that are targeted by FR and YM to glue the Gβγ complex to the inactive Gα-GDP subunit. Unlike all previously developed nucleotide-state specific inhibitors that sequester Gα in its OFF-state but compete with Gβγ, FR and YM actively promote the inhibitory occlusion of Gα-GDP by Gβγ. Through this elegant mechanism, they securely lock the entire heterotrimer, not just Gα, in its inactive state. This discovery identifies FR and YM as molecular glues for Gα and Gβγ that combine simultaneous binding to both subunits with inhibition of G protein signaling.
A German-Swiss collaboration
This research is an example of the value of international scientific collaboration. The work was conducted in the framework of a research consortium funded by the Deutsche Forschungsgemeinschaft (DFG). The team brought together experts from the University of Bonn, Philipps-University Marburg, Goethe University Frankfurt, and Darmstadt University of Technology in Germany, along with the teams at PSI, led by Prof. Schertler from the Center for Life Sciences and Dr. Deupi from the Center for Scientific Computing, Theory, and Data. Each partner in this scientific alliance contributed unique capabilities. The teams in Germany synthesized the FR and YM compounds and characterized their effects in cells, while the PSI researchers focused on obtaining and analyzing the high-resolution three-dimensional structures that revealed the compounds' unexpected mechanism of action. The biotechnology company leadXpro, a spin-off of PSI based at Park Innovaare, helped characterize the G proteins using advanced biophysical methods. "Complex scientific challenges like this require diverse perspectives and specialized skills." explains Prof. Schertler. The PSI teams also provided support to their Frankfurt colleagues who characterized the FR/G protein complex using nuclear magnetic resonance (NMR) methods [4]. "This is truly a case where the whole is greater than the sum of its parts.", notes Dr. Deupi. “Our complementary expertise allowed us to gain insights that wouldn't have been possible through any single approach."
A path to new therapeutic strategies
The discovery of this dual molecular glue mechanism opens exciting new possibilities for drug development. Understanding how these inhibitors work at the molecular level provides insights into drug design for diseases involving dysregulated G protein signaling. The molecular glue approach represents an additional way we can develop drugs targeting cellular communication networks. Rather than simply blocking a single protein, this strategy stabilizes specific conformations of protein complexes. This could lead to more selective drugs with fewer side effects that can target specific signaling pathways involved in cancer and other diseases. Looking ahead, researchers can now use the structural insights from this study to design and synthesize new compounds that preserve the effective molecular glue properties while improving other pharmaceutical characteristics. These next-generation therapeutics may offer hope for patients with conditions where Gq/11 signaling plays a role.
Contact
Original Publication
Mühle, J., Alenfelder, J., Rodrigues, M. J., Jürgenliemke, L., Guixà-González, R., Grätz, L., Andres, F., Bacchin, A., Hennig, M., Schihada, H., Crüsemann, M., König, G. M., Schertler, G., Kostenis, E. & Deupi, X. Cyclic peptide inhibitors function as molecular glues to stabilize Gq/11 heterotrimers. Proceedings of the National Academy of Sciences 122, e2418398122 (2025). doi: 10.1073/pnas.2418398122
Further information
- Voss, J. H., Nagel, J., Rafehi, M., Guixà-González, R., Malfacini, D., Patt, J., Kehraus, S., Inoue, A., König, G. M., Kostenis, E., Deupi, X., Namasivayam, V. & Müller, C. E. Unraveling binding mechanism and kinetics of macrocyclic Gαq protein inhibitors. Pharmacol. Res. 173, 105880 (2021). doi: 10.1016/j.phrs.2021.105880
- Patt, J., Alenfelder, J., Pfeil, E. M., Voss, J. H., Merten, N., Eryilmaz, F., Heycke, N., Rick, U., Inoue, A., Kehraus, S., Deupi, X., Müller, C. E., König, G. M., Crüsemann, M. & Kostenis, E. An experimental strategy to probe Gq contribution to signal transduction in living cells. J. Biol. Chem. 296, 100472 (2021). doi: 10.1016/j.jbc.2021.100472
- Lapadula, D., Farias, E., Randolph, C. E., Purwin, T., McGrath, D., Charpentier, T., Zhang, L., Wu, S., Terai, M., Sato, T., Tall, G. G., Zhou, N., Wedegaertner, P., Aplin, A. E., Aguirre-Ghiso, J. & Benovic, J. L. Effects of Oncogenic Gαq and Gα11 Inhibition by FR900359 in Uveal Melanoma. Mol. Cancer Res. 17, molcanres.0574.2018 (2018). doi: 10.1158/1541-7786.mcr-18-0574
- Bonifer, C., Hanke, W., Mühle, J., Löhr, F., Becker-Baldus, J., Nagel, J., Schertler, G. F. X., Müller, C. E., König, G. M., Hilger, D. & Glaubitz, C. Structural response of G protein binding to the cyclodepsipeptide inhibitor FR900359 probed by NMR spectroscopy. Chem. Sci. 15, 12939–12956 (2024). doi: 10.1039/d4sc01950d