Show filters
New process for stable, long-lasting all-solid-state batteries
PSI researchers have developed a novel process that could make all-solid-state batteries more robust and longer-lasting.
Clean biogas – measurable everywhere
A new analytical method can detect even tiny amounts of critical impurities.
Electric cars and heat pumps can help the Energy Strategy
In future, flexibly operated heat pumps and electric cars could reduce both electricity imports and electricity prices. That is according to a new study by a Swiss research consortium led by ETH Zurich.
Both natural and human emissions shape cloud formation high above Earth
What happens inside the CLOUD chamber?
Particulate pollution re-evaluated
A new study provides data from 43 sites across Europe, showing the respective oxidative stress on the lungs.
Advancing nuclear technologies
NUKEM and Paul Scherrer Institute Sign Memorandum of Understanding to Advance Nuclear Research and Collaboration.
Data for a better vanadium flow
Scientists at PSI have developed a dynamic database on the global vanadium economy. This is meant to advance the use of special energy storage systems – and thus the energy transition.
POLIZERO: PSI project shows paths to climate neutrality
The net-zero target is achievable – if Switzerland sets the right political course now.
Study reveals: Smoke from crop residue burning worsens air pollution in Indian cities
Identifying the main source of air pollution in Indian cities is crucial to reducing the many deaths caused by fine particulate matter (PM₂.₅) – deaths that during the harvest season can account for up to half of all air pollution-related fatalities. An international research team lead by the Paul Scherrer Institute (PSI), funded by the Swiss Agency for Development and Cooperation (SDC) has investigated in detail the sources of the organic components of fine particulate matter in the northern Indian cities of Delhi and Kanpur, located in the Indo-Gangetic Plain. Using novel high-resolution molecular measurement techniques and advanced data analysis, the researchers were able to precisely identify and quantify the sources of organic fine particulate matter.
PSI research at Switzerland’s most-visited museum
Making energy research something visitors can experience: The Swiss Museum of Transport is creating a platform for political and social dialogue on energy issues.
AI paves the way towards green cement
Researchers at PSI are using artificial intelligence to develop environmentally friendly formulations for cement.
Targeted funding of innovation for the energy transition
How do innovations arise and how can they be specifically encouraged for the energy transition? PSI researcher Michael Weinold has been looking into this question using LED lamps as an example.
Water gets in shape for VUV absorption
Nanometre‑thin, free‑flowing liquid sheets now let Swiss Light Source users record pristine VUV absorption spectra of water, and soon any solvent.
A faster route to green hydrogen
The pH value determines how easily hydrogen can be produced from water when cobalt is used as a catalyst. PSI researchers have now found out why.
Net zero: Taking raw materials into account
A new calculation model from PSI illustrates the complex interdependencies between technology, demand for critical raw materials, and environmental impacts on the road to climate neutrality.
Pollutants often originate in the air
In the CLOUD experiment at CERN, PSI researchers have measured with unprecedented precision how harmful organic air pollutants are formed and dispersed.
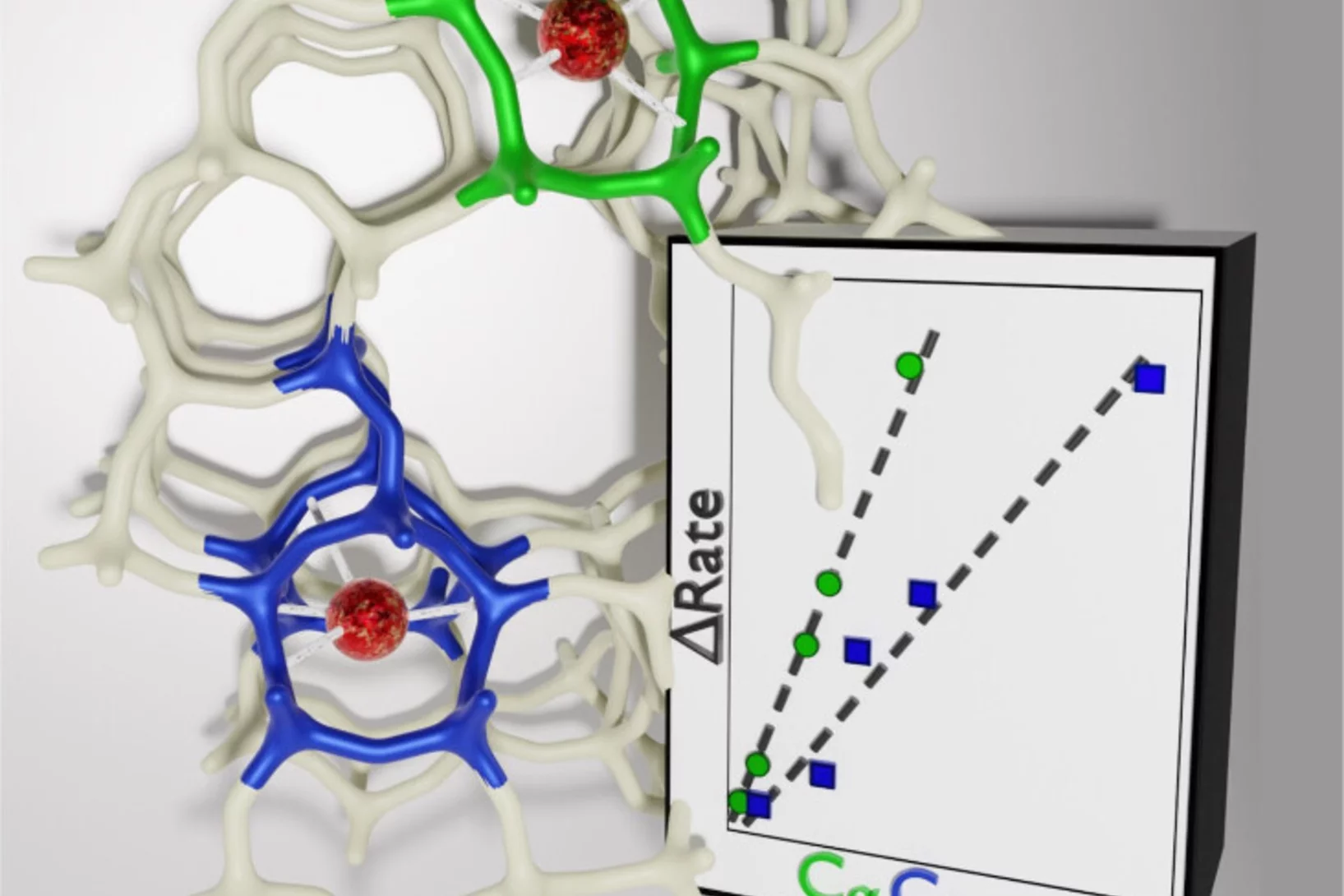
Key reaction steps in the simultaneous conversion of nitrous and nitric oxides on Fe-ZSM-5
After proposing the reaction mechanism of the N2O-NO-SCR reaction on Fe-FER, here we attempted at controlling the Fe speciation on ZSM-5 and at generalizing the result obtained on FER.
New protective coating can improve battery performance
Increasing the energy density of lithium-ion batteries – a sustainable method for cathode surface coating developed at PSI makes it possible.
Moving towards low-carbon road transport
Researchers at the Paul Scherrer Institute PSI have shown how road transport can be decarbonised through the clever integration of renewable energy systems.
Preparing the Future of PSI Large Facilities in Atmospheric Research
The Multiphase Chemistry Group in the Laboratory of Atmospheric Chemistry (LAC) looks back to a nearly 20 years record of activities with in situ X-ray photoelectron spectroscopy (XPS) and in situ scanning transmission X-ray spectromicroscopy (STXM) to address key fundamental questions in atmospheric chemistry. This is the time to consider new horizons, align with current and future needs in atmospheric sciences, and to identify novel opportunities driven by upcoming trends in methods, technologies and facilities. This has been the topic of the Workshop ‘X-ray and Neutron Spectroscopy, Scattering and Imaging in Atmospheric Chemistry’, held at PSI 13 – 15 November 2024.
60 years of the Hotlab
Switzerland’s longest-running nuclear facility, located at PSI, is celebrating its anniversary today.
Getting to the roots of a global health problem
Imad El Haddad analyses the chemical composition and health impacts of particulate matter at the Center for Energy and Environmental Sciences of the Paul Scherrer Institute PSI.
iLab and Synfuels at the Energy Days! at the Swiss Museum of Transport
October 18, 19, and 20, 2024
The iLab from the Paul Scherrer Institute will be part of the Energy Days with exciting workshops. Discover how we can store renewable energy using innovative technologies like Power-to-Gas and drive the energy transition forward.
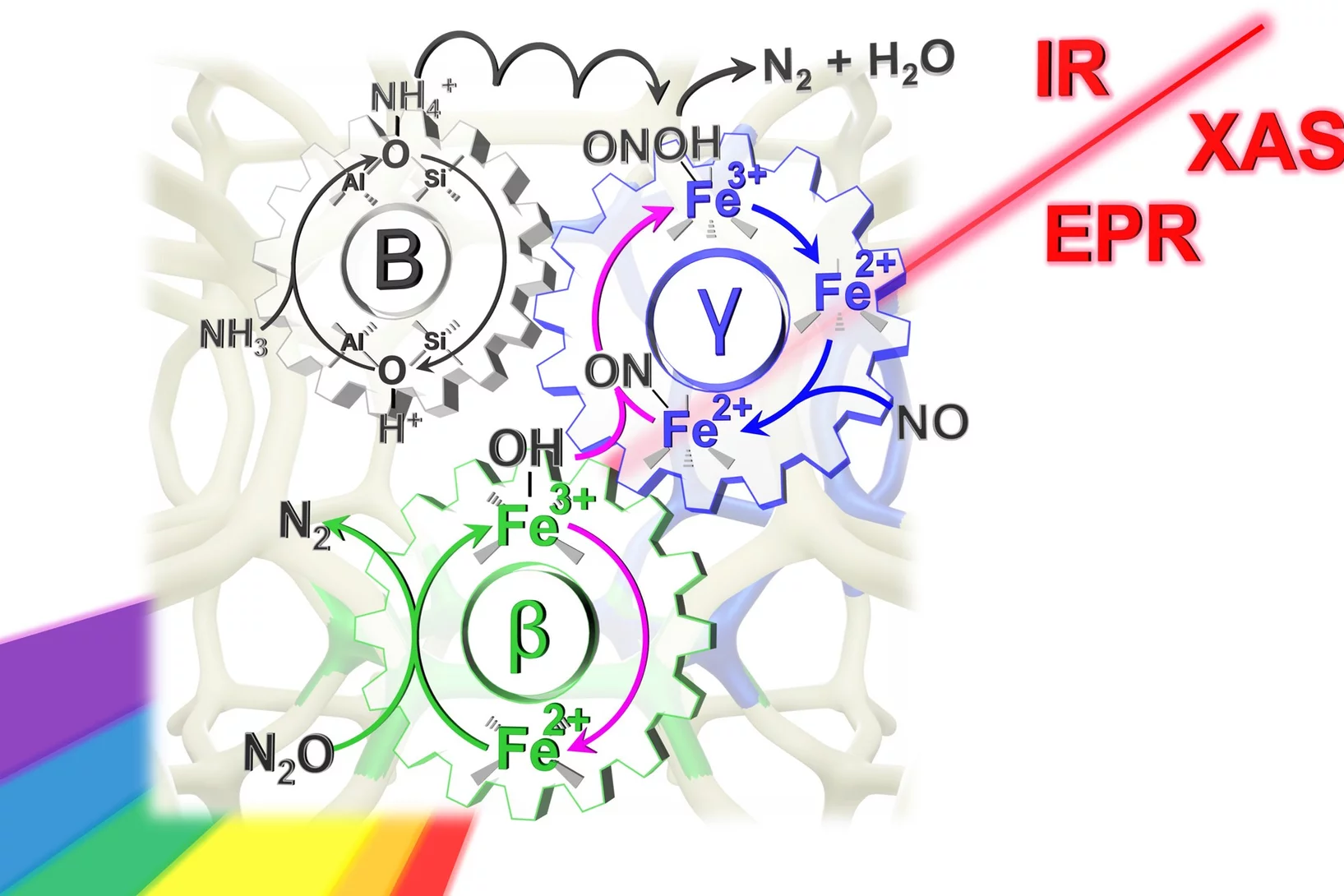
Dual-site reaction mechanism for the simultaneous reduction of nitrous and nitric oxides
We have applied three spectroscopic techniques (XAS, EPR and DRIFTS) in combination withe modulated excitation and catalytic data to decipher and propose the complete reaction mechanism of the simultaneous reduction of N2O and NO
How catalysts remove dangerous nitrogen oxides
In industrial catalysis, iron is not equal to iron.
Artificial intelligence explores the underground
The properties of geological units are determined using images of drill cores.
Fast as a plane, clean as a train
In a collaboration across Switzerland, researchers have evaluated the potential environmental impacts of so-called hyperloop systems.
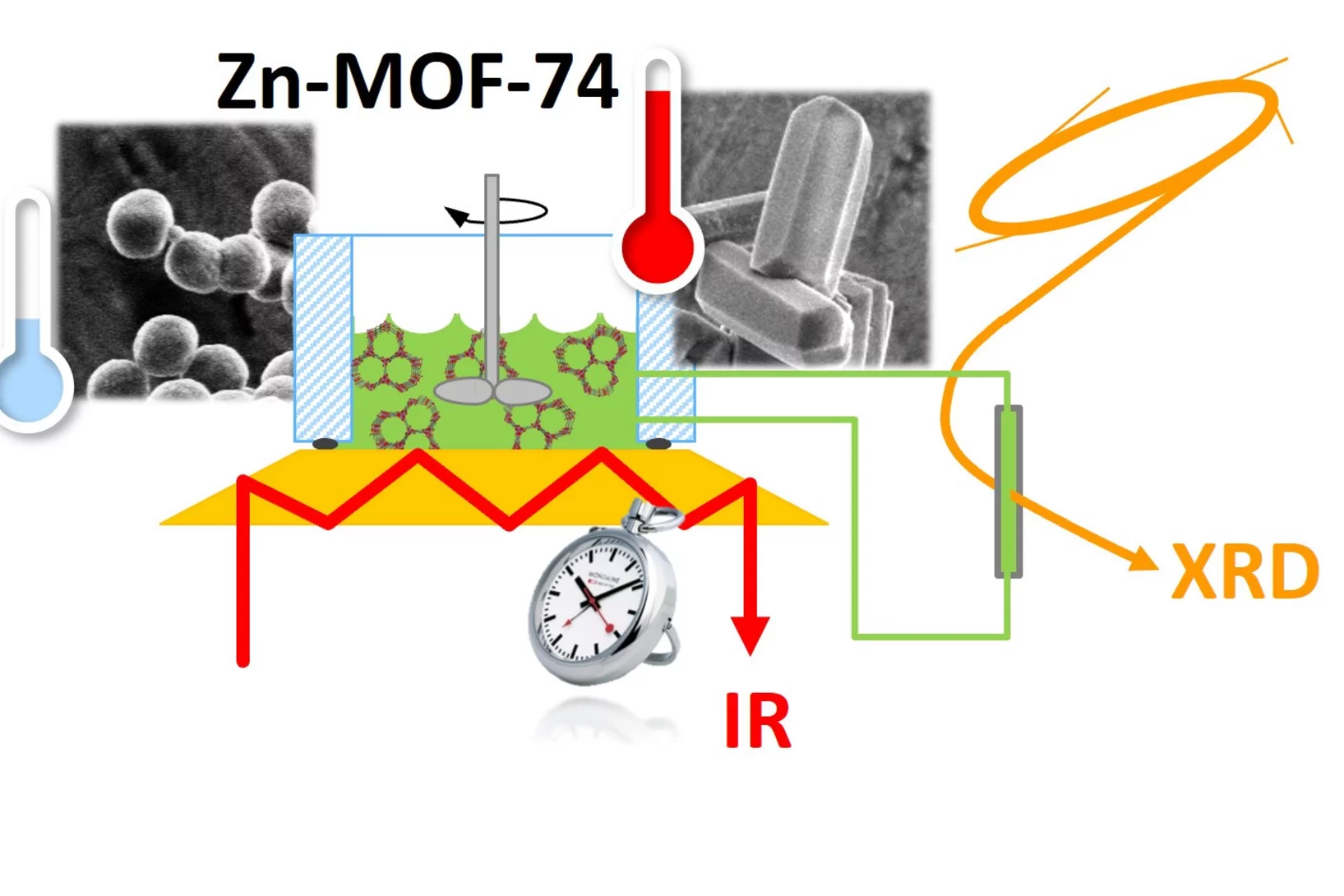
Uncovering the secrets of rapid and green metal-organic framework synthesis
Using ATR-IR spectroscopy and high-energy XRD, we have studied the mechanism of ambient temperature aqueous synthesis of Zn-MOF-74. Starting from the need to change Zn precursor to disentangle signals in ATR-IR spectroscopy, we also developed a rapid synthesis of Zn-MOF-74 with Zn perchlorate.
Carbenium ions in the methanol-to-olefins process
The methanol-to-olefins process converts methanol into hydrocarbons over zeolite-based catalysts following a dual-cycle reaction mechanism within the paradigm of an hydrocarbon pool chemistry. In this work, we use operando DRIFTS/GC to correlate the nature of species residing within the porosity of zeolites (DRIFTS) and the products distribution (GC).
Hydrogen Electrode for Membrane Water Electrolyzers with Low Gas Crossover
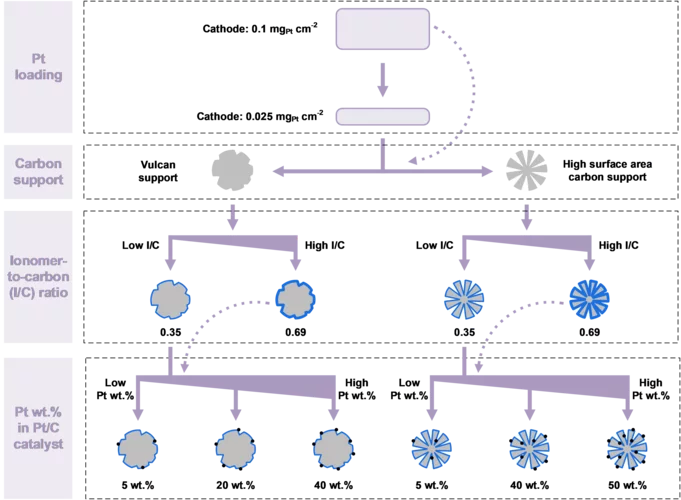
Proton exchange membrane (PEM) water electrolyzer are considered a for the Energy Transition to produce green hydrogen for fuel cell-based mobility, industrial processes, and seasonal storage. Platinum group metals (PGMs) are conventionally used as catalysts for electrode reactions due to their outstanding catalytic activity and chemical stability in the harsh acidic environment of the cell. Commercial carbon-supported platinum (Pt/C) electrocatalysts remains a state-of-the-art choice for the hydrogen evolution reaction (HER) on the cathode side of the cell. While a high Pt loading between 0.5 and 1.0 mgPt/cm2 is commonly used today, a reduction of the Pt loading to below 0.05 mgPt/cm2 is desired to reduce the cost of PGM usage in megawatt-scale PEM water electrolysis systems. In addition, in connection with the trend towards the use of thinner membranes (<0.1 mm), gas crossover through the membrane from the cathode to the anode side can lead to the formation of an explosive gas mixture in the anode product stream. In this study, we varied the design parameters for the cathode catalyst layer to reduce the Pt loading to 0.025 mg/cm2 while at the same time minimizing the rate of hydrogen crossover to the anode.
Where should hydrogen be produced in the future?
Researchers at PSI have been looking into where the hydrogen for a future hydrogen economy should be produced and what impact this energy carrier will have on the environment.
Sources of smog in Beijing identified
Researchers at PSI are investigating the wide range of causes underlying smog in Beijing.
Sustainable aviation fuel from the PSI campus
In collaboration with climate start-up Metafuels, a pilot plant for the production of sustainable aviation fuel is being set up on the PSI campus.
Digitalisation: a blessing for the energy transformation
Researchers at PSI have calculated the influence of digitalisation on energy consumption.
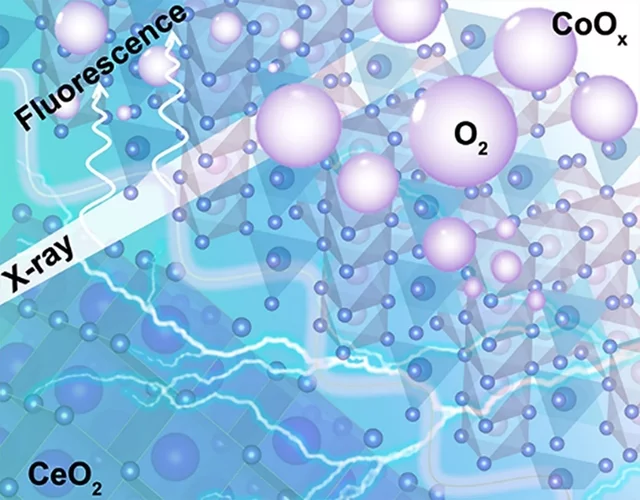
Insights into the superior oxygen evolution reaction activity of CoOx/CeO2 composite electrocatalyst
CeO2 significantly enhances the oxygen evolution reaction (OER) activity of CoOx, although the mechanism behind this synergy is still unclear. Here, operando hard X-ray absorption spectroscopy (hXAS) is applied to monitor the Co-K edge and Ce L3 edge in CoOx/CeO2 to shed light on the evolution of Co and Ce oxidation states during OER. In addition, ex situ soft XAS (sXAS) characterizations provide information on the irreversible surface-specific transformations of the Co L3 edge as well as the O K edge.
Real-Time Insights into Sodium-Ion Battery Chemistry
Identification of gaseous decomposition products from irreversible side-reactions enables understanding of inner working of rechargeable batteries. Unlike for Li-ion batteries, the knowledge of the gas-evolution processes in Na-ion batteries is limited. Our study revealed that Na-ion cells develop a less stable solid-electrolyte interphase (SEI) compared to Li-ion cells due to higher solubility of SEI constituents in Na-electrolytes.
Can aerosols stop global warming?
Injecting particles into the stratosphere to cool the earth? In our interview, PSI researcher Markus Ammann comments critically on the controversial subject of solar geoengineering.
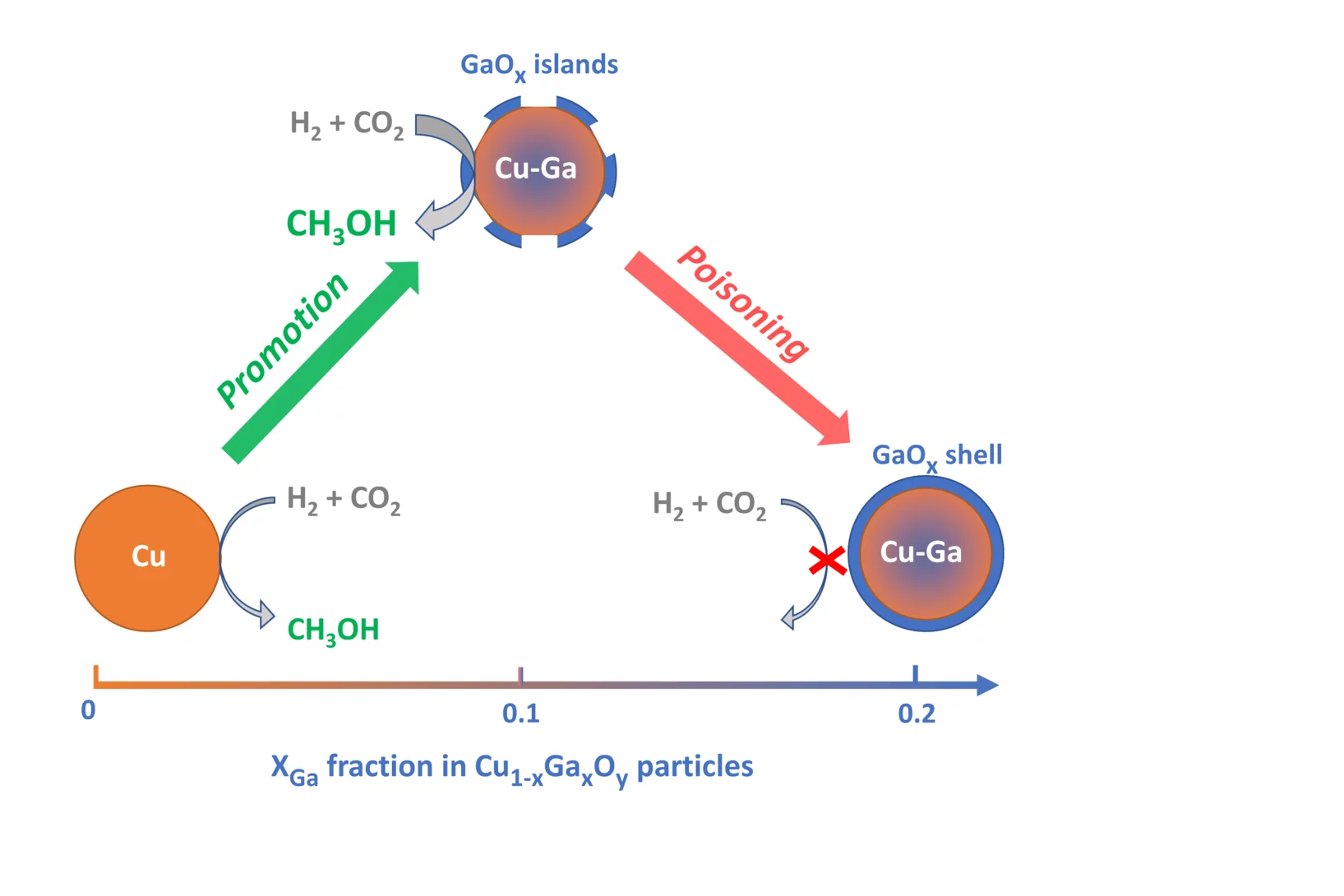
Promotion versus Poisoning in Copper–Gallium-Based CO2-to-Methanol Hydrogenation Catalysts
Cu–Ga-based CO2-to-methanol hydrogenation catalysts display a range of catalytic performance, depending on their preparation. Here, we investigated how the Ga/Cu ratio and Ga speciation affect the catalytic activity. Using surface organometallic chemistry, we prepared a series of silica-supported 3–6 nm Cu1–xGaxOy nanoparticles with a range of xGa. The materials display a volcano-type activity behavior, where methanol formation is promoted when xGa < 0.13–0.18 and is suppressed at higher values, indicating a poisoning of the catalysts. In situ X-ray absorption spectroscopy and in situ infrared spectroscopy helped to understand the structure-activity relationship.
How to clean up New Delhi’s smoggy air
PSI researchers are tracking down pathogenic aerosols in India.
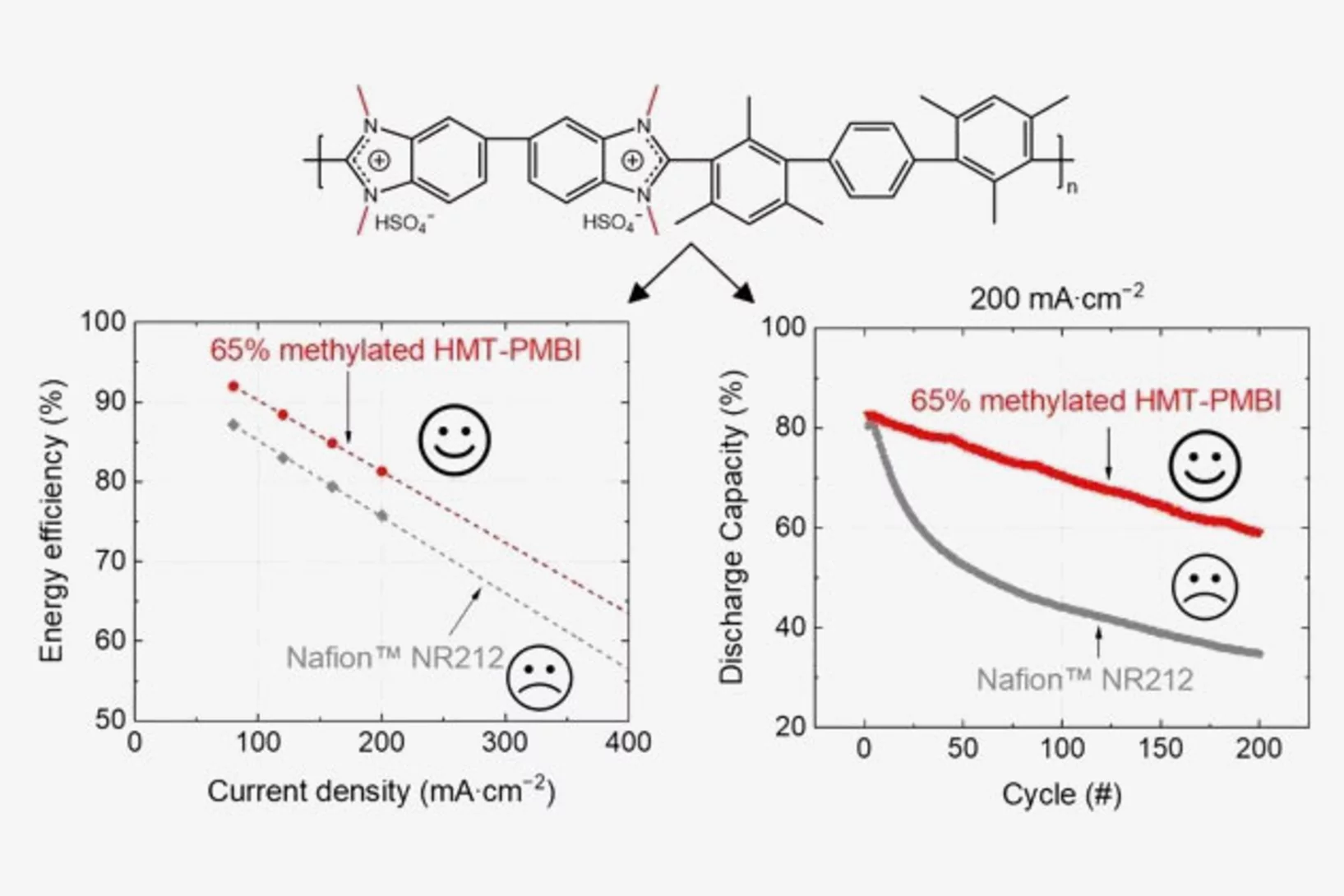
Polybenzimidazole Membrane Design Principles for Vanadium Redox Flow Batteries
Energy storage technologies with long storage duration are essential to stabilize electricity grids with a high share of intermittent renewable power. In a redox flow battery, the electrochemical conversion unit, where the charging and discharging reaction takes place, is spatially separated from the energy storage medium. In the all-vanadium redox flow battery (VRFB), a sulfuric acid aqueous electrolyte with dissolved vanadium ions is used as the storage medium. Vanadium is present in 4 different oxidation states, the redox couple vanadium(II) and (III) on the negative side of the cell, and vanadium(IV) and (V) on the positive side. This allows the battery to be repeatedly charged and discharged. A separator or membrane is used between the negative and positive electrode, which should selectively conduct the ions of the supporting electrolyte and minimize the passage of vanadium ions. Fluorinated membranes, such as Nafion™, are often used for this key component, but these ionomers were not originally developed for this application and therefore have functional shortcomings. Furthermore, the production and use of fluorinated materials is to be severely restricted or even banned in Europe. Therefore, the development of hydrocarbon-based membranes for the VRFB is of great importance. The study reported here focuses on polybenzimidazole polymers and membranes, which could be a promising materials class for next generation flow batteries.
Is climate-neutral air travel possible?
Air transport too is to become climate-neutral – how can sustainable fuels, like those developed at PSI, contribute to this?
Unraveling degradation processes in a bipolar membrane CO2 electrolyzer by time-resolved X-ray tomographic microscopy
Employing a bipolar ion conducting membrane (BPM) in forward bias is a convenient solution for the biggest issues in the more common anion exchange membrane (AEM) CO2 co-electrolysis: the degradation of the performance caused by carbonate salt precipitation at the cathode and the decrease of net CO2 conversion caused by the crossover of this molecule from cathode to anode also requiring energy for downstream gas separation. However, the performance and stability of this device remain largely insufficient when using such a BPM configuration. To understand the reasons for this, we performed time-resolved X-ray tomographic microscopy of an operating BPM CO2 electrolyzer. The imaging method reveals partly unexpected degradation processes that result in design recommendations for improvement.
Insights into the superior oxygen evolution reaction activity of CoOx/CeO2 composite electrocatalyst
CeO2 significantly enhances the oxygen evolution reaction (OER) activity of CoOx, although the mechanism behind this synergy is still unclear. Here, operando hard X-ray absorption spectroscopy (hXAS) is applied to monitor the Co-K edge and Ce L3 edge in CoOx/CeO2 to shed light on the evolution of Co and Ce oxidation states during OER. In addition, ex situ soft XAS (sXAS) characterizations provide information on the irreversible surface-specific transformations of the Co L3 edge as well as the O K edge.
Glacier melting destroys important climate data archive
PSI researchers analysed two ice cores from the Corbassière glacier on Grand Combin.
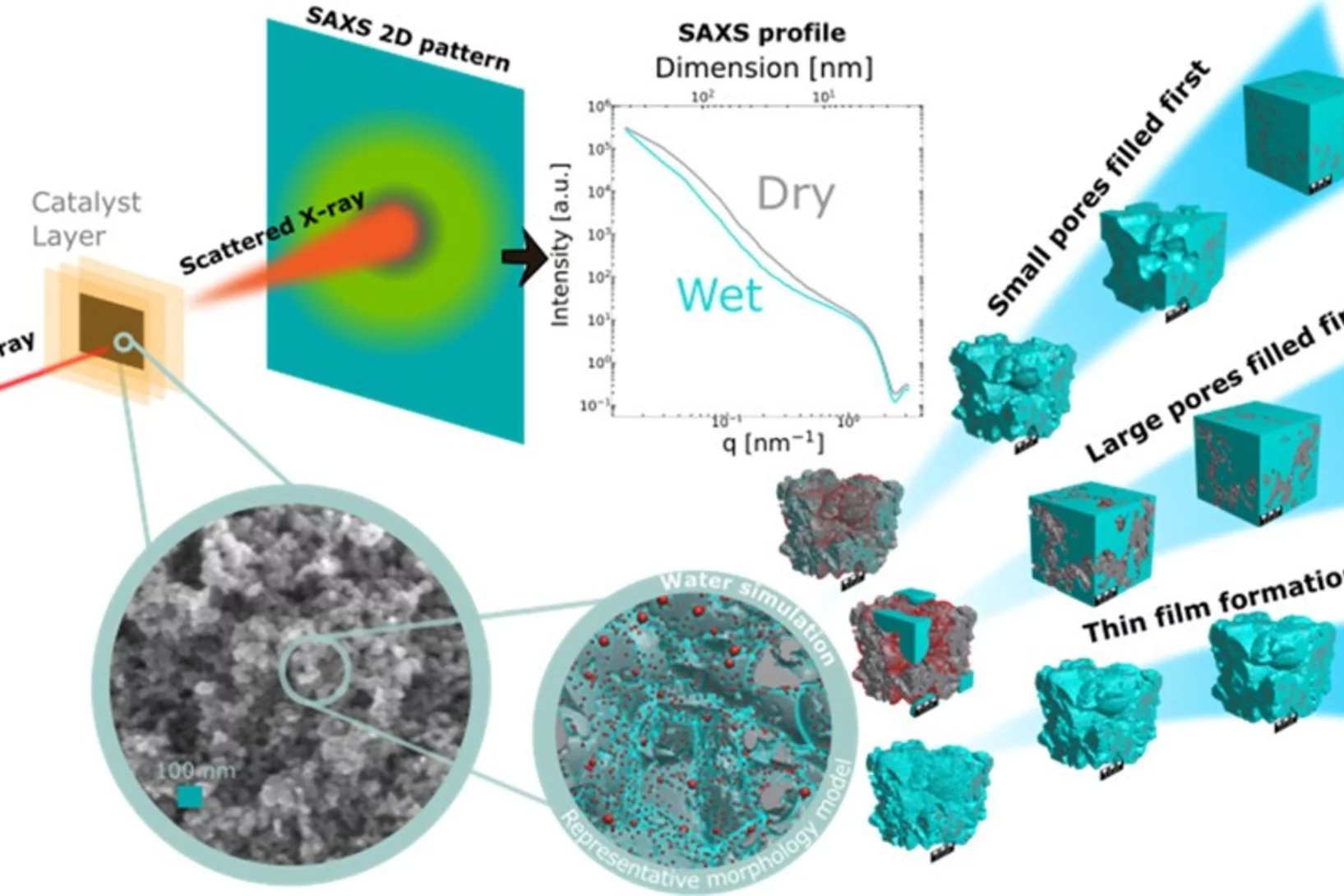
Quantification of PEFC Catalyst Layer Saturation via Small-Angle X‑ray Scattering
The complex nature of liquid water saturation in polymer electrolyte fuel cell (PEFC) catalyst layers (CLs) greatly affects the device performance. To investigate this problem, a method to quantify the presence of liquid water in a PEFC CL using small-angle X-ray scattering (SAXS) was developed in a collaboration of researchers of the Federal Institute for Materials Research and Testing (BAM, Berlin, Germany), the Photon Science Division and the Electrochemistry Laboratory of PSI. The method leverages the differences in electron densities between the solid catalyst matrix and the CL-pores filled with liquid water under dry and wet conditions, respectively.
How important is hydrogen for the energy transition?
Assessments by PSI energy expert Thomas J. Schmidt
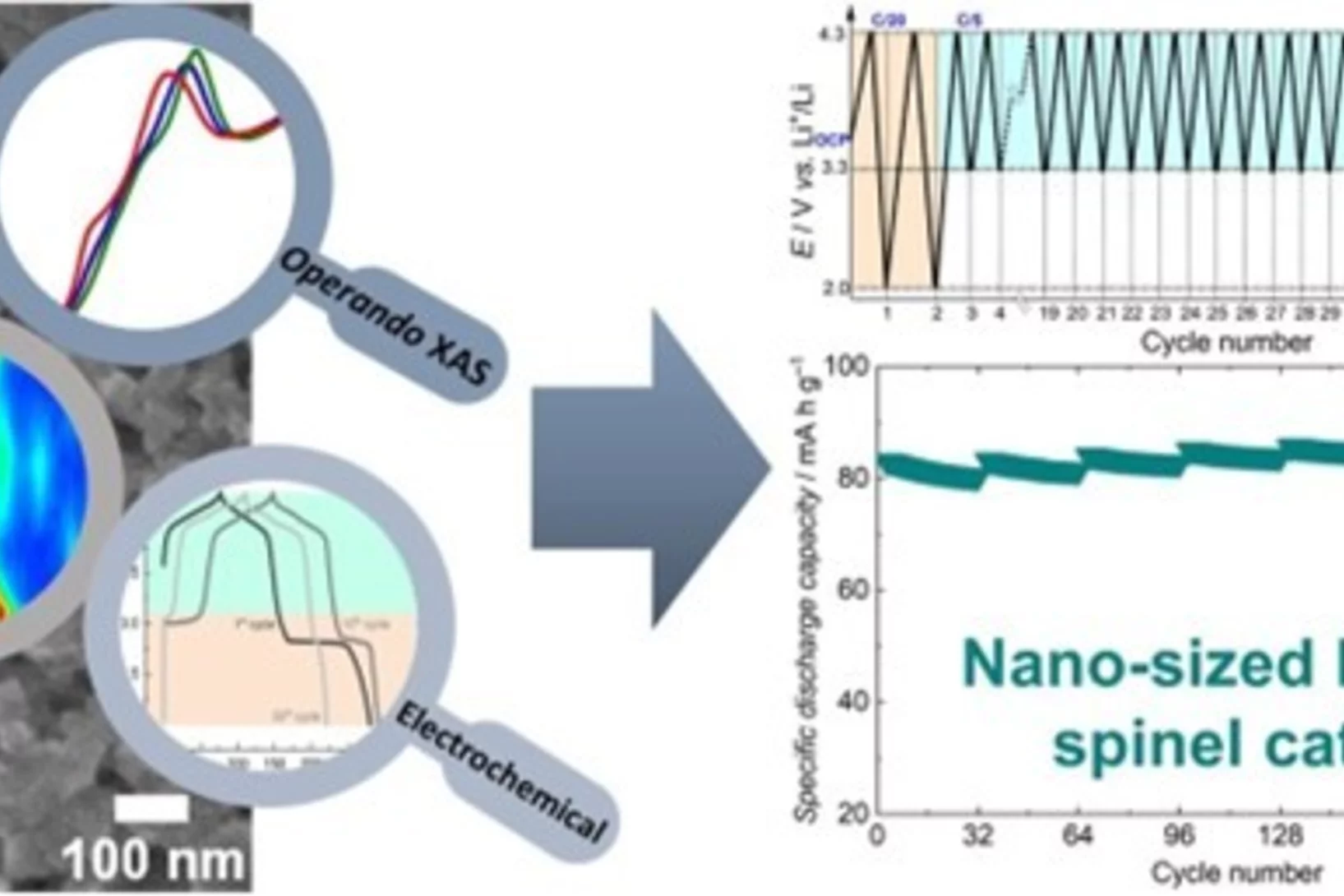
Understanding the (de-)lithiation mechanism of nano-sized LiMn2O4 allows achieving long-term cycling stability
We report an in-depth investigation of the local atomic geometry, electronic and crystallographic structure evolution of nano-sized LiMn2O4 using operando XAS and XRD to shed light on (de-)lithiation mechanism when cycled in wide voltage range of 2.0 to 4.3 V vs Li+/Li. Leveraging on these findings, a novel electrochemical cycling protocol, with periodic deep discharge, yields superior electrochemical performance cycled in the range of 3.3 to 4.3 V exhibiting an excellent structure cyclability and an unprecedented increase in the specific capacity upon long cycling.
Better batteries for electric cars
PSI researchers make physical and chemical changes in batteries visible.