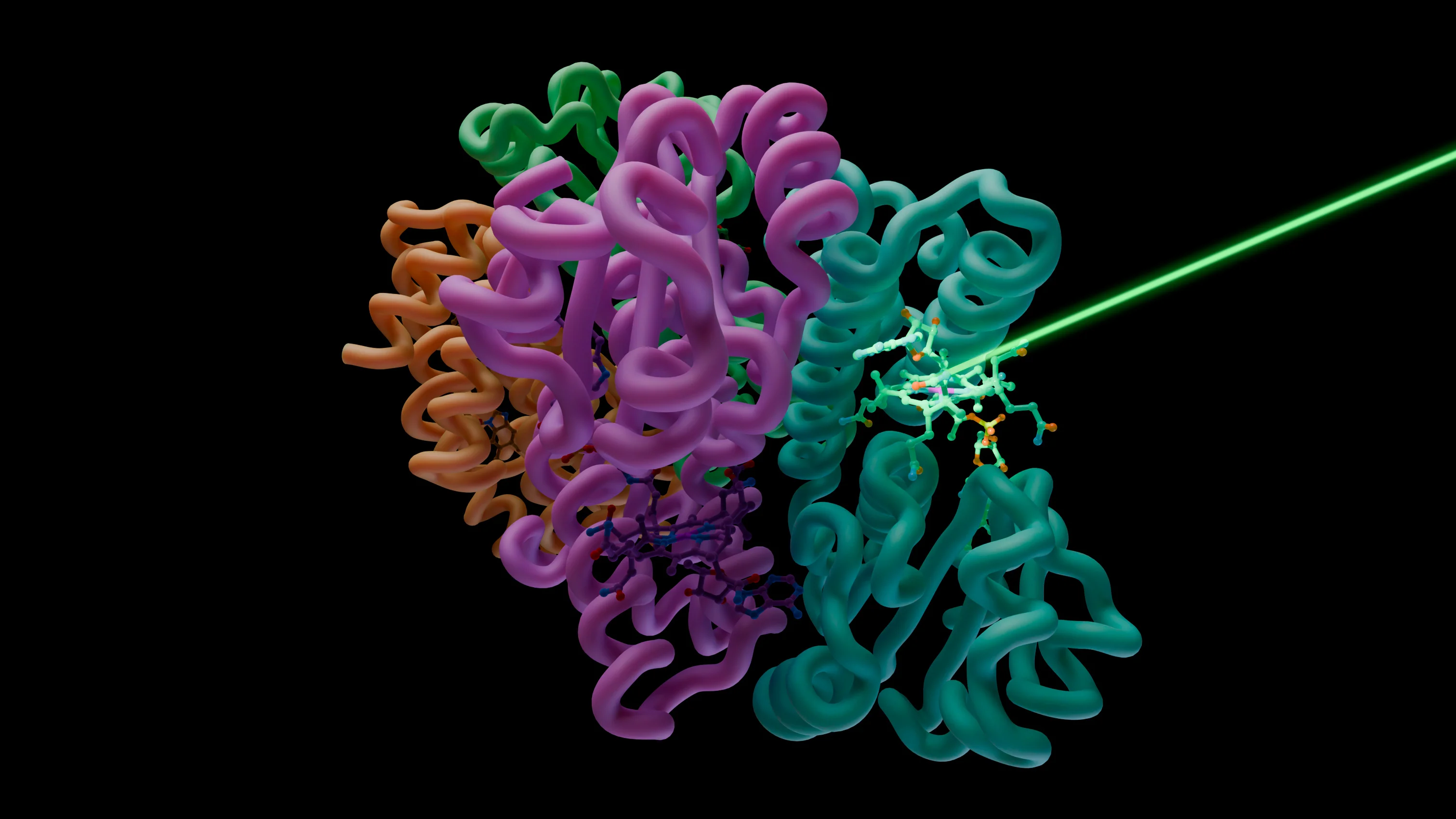
Using X-ray free-electron lasers and synchrotron light at facilities in Switzerland, Japan, France and the UK, a worldwide collaboration of scientists have discovered how a vitamin B12-based photoreceptor works. Understanding how photoreceptors function aids future technological applications, such as optogenetics, that involve controlling cellular processes with light. The findings are published in Nature.
Vitamin B12 is an organometallic cofactor found in many enzymes that control essential processes in various organisms, including humans. It came as a surprise a decade ago that vitamin B12 derivatives had been repurposed for light sensing by a large family of previously unknown photoreceptors in bacteria that fulfil various functions.
The prototypical B12 photoreceptor CarH, for example, regulates the expression of genes involved in protecting bacteria against excess sunlight. It achieves this by binding to DNA in the dark, acting as a molecular doorstop. Upon illumination, its tetrameric architecture breaks apart, enabling transcription by unbinding from DNA.
The way in which this and other B12 photoreceptors function at a molecular level has remained a mystery ever since. However, an international consortium led by scientists at the Institut de Biologie Structurale in Grenoble, France has now combined experimental techniques using X-ray free-electron lasers at the Paul Scherrer Institute PSI in Switzerland (SwissFEL) and Japan (SACLA), as well as the synchrotrons in France (ESRF) and the UK (Diamond Light Source), with quantum-chemical calculations to uncover the inner workings of CarH.
“At SwissFEL, we combined the brilliance of the X-ray free-electron laser with the longer time delays enabled by the fixed-target sample delivery systems at the Cristallina endstation to understand how CarH changes from the microseconds to tens of milliseconds at high resolution,” says John Beale from the PSI Center for Photon Sciences. “Combining these data with complementary insights from other light sources, we could piece together a complete picture of the molecular events of photoactivation.”
Observing the photoreceptor’s structural change in real time
After triggering photoactivity in CarH with an intense pulse of visible laser light, the researchers observed the photoreceptor's structural changes in real time. From the first moments after light absorption on the nanosecond timescale to the timescale at which the photoreceptor’s tetrameric architecture is lost, the study reveals the sequence of orchestrated molecular events that underpin its function.
Stunningly, the researchers discovered an unanticipated intermediate state that the photoreceptor transiently adopts during the reaction process. This state is hypothesised to protect the photoreceptor from reverting to its initial dark state and to direct it to progress in the reaction. Such an intermediate state has not been found in vitamin B12-containing enzymes, which makes it a plausible explanation for the light-sensing capability of vitamin B12 photoreceptors.
Understanding how CarH functions at a molecular level will enable this photoreceptor to be modified for biotechnological applications, such as optogenetics, which involves controlling cellular processes with light.
Contact
Dr. John Henry Beale
PSI Center for Photon Science
Paul Scherrer Institute PSI
+41 56 310 47 15
john.beale@psi.ch
Original publication
-
Rios-Santacruz R, Poddar H, Pounot K, Heyes DJ, Coquelle N, Mackintosh MJ, et al.
Integrated structural dynamics uncover a new B12 photoreceptor activation mode
Nature. 2026. https://doi.org/10.1038/s41586-025-10074-2
DORA PSI