Researchers from the Paul Scherrer Institute (PSI) and the Hungarian Academy of Sciences join forces to investigate the basic properties of argillaceous rocks in a repository for high-level radioactive waste. As the results reveal, the insights gained so far for Opalinus Clay can be transferred also to the Boda Clay found in Hungary. These two types of clay formations are regarded as potential host rocks for a repository by the respective countries. Besides the new knowledge obtained, the project also served as an exchange of expertise.
In the worldwide search for deep repositories for high-level radioactive waste, geologically stable, virtually impermeable rocks are considered. In this context, natural barriers (host rocks) as well as technical safety barriers will be used to reduce the mobility of the radionuclides present in the waste and ensure maximum safety over long periods. The less mobile radionuclides are, the greater the proportion of them that will decay in the rock and hence not reach the biosphere.
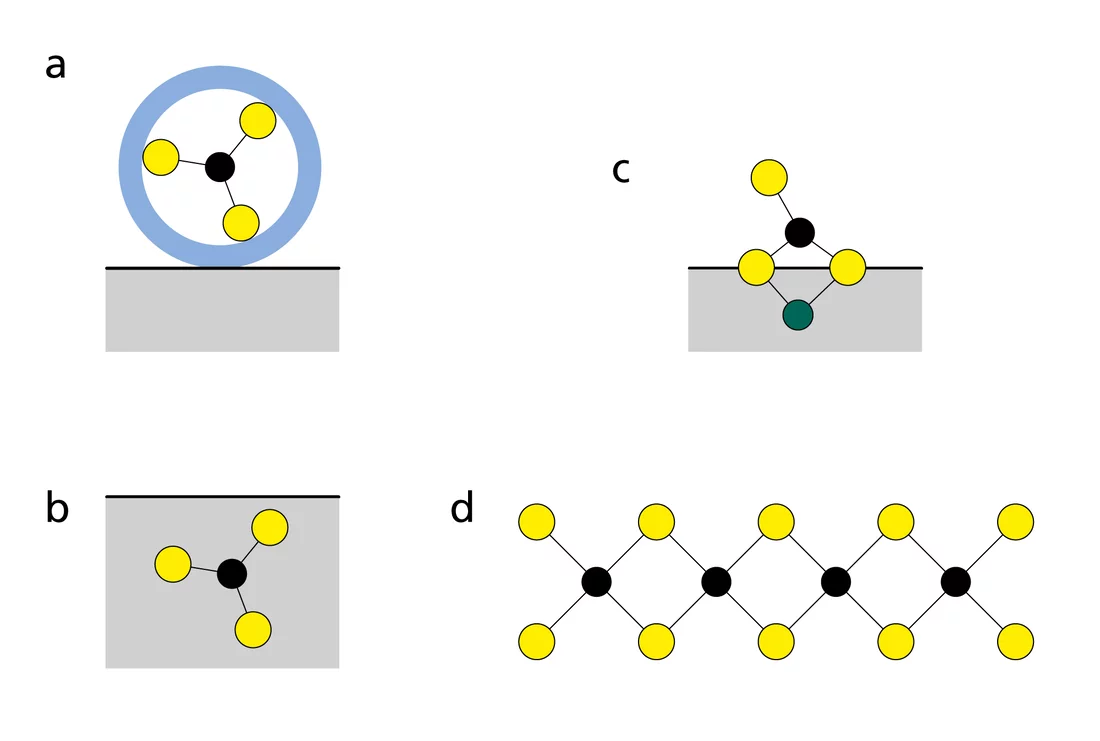
In order to predict the mobility of radionuclides, researchers must understand the processes that control the uptake of radionuclides in rocks at the molecular level. For example, radionuclides which bind to the rock by electrostatic attraction are more mobile than those that are adsorbed by chemical bonds, incorporated into the rock or even form new solids (Fig. 1). Previous laboratory and synchrotron-based studies at PSI showed that radionuclides sorb particularly strongly on argillaceous rocks. The reason for this is that the majority of radionuclides are positively charged and form strong bonds with the negatively charged surfaces of the argillaceous rock.
Opalinus Clay and Boda Clay: a comparison
In Switzerland, the search for a site for deep geological disposal of high-level radioactive waste focuses on locations with clay-rich rocks, so-called argillaceous rocks. In collaboration with the National Cooperative for the Disposal of Radioactive Waste (NAGRA), PSI has been researching such argillaceous rocks for more than ten years.
While Switzerland has opted for the Opalinus Clay, the Boda Clay is the first choice in Hungary. Both host rocks consist largely of minerals from the class of phyllosilicates, which are grouped together under the name of illite. They are made up of three-layer clay minerals, consisting of an aluminum octahedral layer, surrounded by two silicate tetrahedral layers.
Both Opalinus and Boda Clay are sedimentary rocks, the Opalinus Clay being a marine sediment, and the Boda Clay a lake sediment. This results in differences between the two rock types. For instance, Boda Clay contains the iron mineral hematite which gives the rock its red to reddish brown color.
PSI researchers teamed up with colleagues from Hungary to examine how far Boda Clay and Opalinus Clay have the same uptake properties, i.e. whether they retain the same radionuclides equally as effectively. The project arose from the more fundamental question as to which minerals in argillaceous rocks are responsible for the uptake of radionuclides
Sorption model put to the test
The experiments on complex rocks should serve as a touchstone for a theoretical model of the radionuclide adsorption on the mineral illite developed by PSI researchers. Using their model, the PSI researchers attempt to understand how single minerals in the Opalinus Clay interact with the radionuclides. From this, they then extrapolate how many of which radionuclides the rock will adsorb. In other words, they go from the bottom level of the minerals to the top level of the more complex rocks – which scientists refer to as a bottom-up approach.
The scientists used different techniques to study the adsorption behaviour of radionuclides on Boda Clay and Opalinus Clay. Firstly, radionuclides that are relevant for a repository were brought into contact with argillaceous rock in an aqueous solution and after reaching an equilibrium the suspension was centrifuged to separate the aqueous solution from the solid rock. By measuring how many of the radionuclides remained in the solution, the number of radionuclides that were still retained to the rock could be determined. The amount adsorbed corresponded well to the values predicted by the PSI model. Only elements such as zinc, cobalt and nickel adsorbed more than the model predicted when their initial concentrations were high. By means of the technique of X-ray absorption spectroscopy, which is available at synchrotron facilities such as the Synchrotron Light Source SLS at PSI, the researchers found that this surplus, that would only increase the safety of the repository, was due to precipitation. This means that elements such as zinc were extracted from the aqueous solution by undergoing a transition into the solid state and were thus also retained in the rock.
In addition, studies have shown that the uptake of metals such as nickel and zinc (called divalent transition metals) takes place at the edges of the clay particles (Fig. 2). Furthermore, X-ray absorption spectroscopy has clearly demonstrated that these transition metals could become incorporated in the clay structure in the long term.
One more method made use of X-ray radiation from a synchrotron facility. The researchers used a fluorescence technique, in which the X-rays are focussed down to micrometers, in order to find out which radionuclides are correlated to mineral phases in the argillaceous rock. As every element has a characteristic fluorescence spectrum, the elements sorbed to specific mineral phases could be identified and their concentrations determined. Finally, the researchers resorted to another spatially resolved technique: micro-X-ray diffraction. With the aid of this technique they concluded that the clay minerals were responsible for the bulk of the adsorption in both Opalinus Clay and Boda Clay.
PSI model passes test
The latest experiments also confirm the accuracy of the PSI researcher’s bottom-up model for other types of argillaceous clay like Boda Clay. The tests reveal that the clay minerals, especially illite, are the main sink for the adsorption of radionuclides in argillaceous rock, regardless of whether it is Opalinus Clay or Boda Clay. “This increases the confidence in our model and shows that it can be used in safety analyses for a repository beyond Switzerland’s borders,” says the PSI researcher Bart Baeyens, who co-developed the sorption model. Rainer Dähn, who was also involved in the project, highlights the transfer of knowhow achieved: “The colleagues from Hungary brought their expertise in X-ray fluorescence and X-ray diffraction to the table, we added our experience in wet chemical and X-ray absorption techniques at synchrotron sources and both sides learned from each other.”
Text: Paul Scherrer Institut/Leonid Leiva
Additional information
This project was funded in the framework of the Swiss contribution to EU enlargement (SH 7/2/11)http://www.contribution-enlargement.admin.ch/en/Home/Countries/Hungary
Contact
Dr. Bart Baeyens, Laboratory for Waste Management, Paul Scherrer Institut,Telephone: +41 56 310 43 16, E-Mail: bart.baeyens@psi.ch
Dr. Rainer Dähn, Laboratory for Waste Management, Paul Scherrer Institut,
Telephone: +41 56 310 21 75, E-mail: rainer.daehn@psi.ch