Abstract:
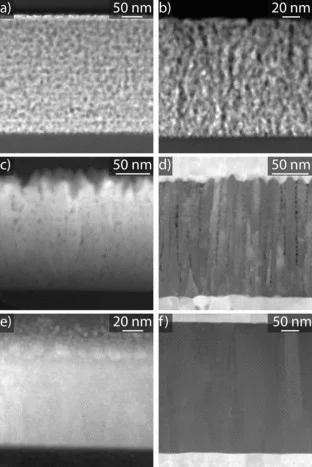
The electrical conductivity of dense and nanoporous zirconia-based thin films is compared to results obtained on bulk yttria stabilized zirconia (YSZ) ceramics. Different thin film preparation methods are used in order to vary grain size, grain shape, and porosity of the thin films. In porous films, a rather high conductivity is found at room temperature which decreases with increasing temperature to 120 °C. This conductivity is attributed to proton conduction along physisorbed water (Grotthuss mechanism) at the inner surfaces. It is highly dependent on the humidity of the surrounding atmosphere. At temperatures above 120 °C, the conductivity is thermally activated with activation energies between 0.4 and 1.1 eV. In this temperature regime the conduction is due to oxygen ions as well as protons. Proton conduction is caused by hydroxyl groups at the inner surface of the porous films. The effect vanishes above 400 °C, and pure oxygen ion conductivity with an activation energy of 0.9 to 1.3 eV prevails. The same behavior can also be observed in nanoporous bulk ceramic YSZ. In contrast to the nanoporous YSZ, fully dense nanocrystalline thin films only show oxygen ion conductivity, even down to 70 °C with an expected activation energy of 1.0 ± 0.1 eV. No proton conductivity through grain boundaries could be detected in these nanocrystalline, but dense thin films.
Keywords: Solid oxide fuel cells; YSZ; Nanoporous thin films; Proton conductors; Oxygen ion conductivity; Aerosol-assisted chemical-vapor-deposition; Spray pyrolysis;
Facility: ENE, ETHZ, LMX, Thin Films and Interfaces
Reference: Barbara Scherrer, Meike V.F. Schlupp, Dieter Stender, Julia Martynczuk, Jan G. Grolig, Huan Ma, Peter Kocher, Thomas Lippert, Michel Prestat, and Ludwig J. Gauckler , Adv. Funct. Mater. 23, 1957–1964 (2013)
Read full article: here
Facility: ENE, ETHZ, LMX, Thin Films and Interfaces
Reference: Barbara Scherrer, Meike V.F. Schlupp, Dieter Stender, Julia Martynczuk, Jan G. Grolig, Huan Ma, Peter Kocher, Thomas Lippert, Michel Prestat, and Ludwig J. Gauckler , Adv. Funct. Mater. 23, 1957–1964 (2013)
Read full article: here