Photocatalytic Thin Films for Water Splitting
Project co-financed by a grant from Switzerland through the Swiss Contribution to the enlarged European Union.
Photocatalytic water splitting to harvest hydrogen is very attractive for renewable energy production in order to become independent of fossil fuels sources and thereby reducing the carbon dioxide emission. The idea of water splitting for a clean hydrogen production is based on a photocatalyst in aqueous solution irradiated by sunlight. Several materials (e.g. TiO2) show photocatalytical activity using only a small part (UV) of the solar spectrum, due to the relatively large energy gap (3.2eV for TiO2). Nitrogen substitution into the oxygen side reduces the band gap, which expands the usable spectrum towards visible light. Some examples are perovskite-based oxynitrides, such as LaTiO3-xNx, BaTaO3-xNx, and CaTaO3-xNx.
The objective is to prepare thin films of less expensive and readily available metal oxynitrides, which show photocatalytical activity under solar radiation. The deposition of the thin films is done using a modified setup for pulsed laser deposition (PLD). A reactive gas pulse (e.g. NH3) is used in the modified PLD setup, also called Pulsed Reactive Crossed-Beam Laser Ablation (PRCLA), to dope the thin film with nitrogen. Thin films as model systems have the added benefit that structural, crystallographic and surface property can be more readily correlated with photocatalytic activities.
The objective is to prepare thin films of less expensive and readily available metal oxynitrides, which show photocatalytical activity under solar radiation. The deposition of the thin films is done using a modified setup for pulsed laser deposition (PLD). A reactive gas pulse (e.g. NH3) is used in the modified PLD setup, also called Pulsed Reactive Crossed-Beam Laser Ablation (PRCLA), to dope the thin film with nitrogen. Thin films as model systems have the added benefit that structural, crystallographic and surface property can be more readily correlated with photocatalytic activities.
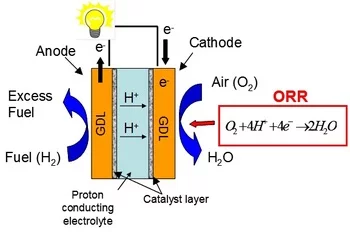
Electrocatalysis
The oxygen reduction reaction (ORR) occurring at the cathode side of polymer electrolyte fuel cells (PEFCs) suffers from poor kinetics, resulting in overpotentials of about 0.3 to 0.4 V. Pt-based catalysts are still recognized as the materials of choice for PEFC cathodes and in the last decades several efforts have been devoted to maximize its catalytic activity towards ORR. According to previous studies, the main possibilities for increasing the ORR kinetics are (i) reducing the interatomic Pt-Pt bond distance and hence modify the physical structure of Pt (Jalan 1983), (ii) shifting the d-band center and in turn influence the electronic structure (Nørskov, Rossmeisl et al. 2004), (iii) increasing the active surface area of the electrocatalyst (Mukerjee 1990) or (iv) tailoring the Pt(hkl) surface and hereby manipulate the specific adsorption of species from the electrolyte (Schmidt, Stamenkovic et al. 2001).
The main goal of the present work is to gain further understanding of the relation between Pt interatomic distance and surface termination and its electrocatalytic activity towards ORR. These properties can be studied on thin films. Pulsed laser deposition represents a suitable technique for fabricating thin Pt films for electrocatalysis of varying crystalline orientation and strain.
A novel class of Pt-based catalyst will be developed by fabricating epitaxial thin films of Pt on single crystal strontium titanate (STO) using pulsed laser deposition. Due to the structural similarities (e.g. lattice parameters) of the substrates and Pt, a heteroepitaxial growth of a strained, thin Pt film is expected. The effect of the strain induced by the substrate will be investigated in terms of Pt interatomic distance and electronic structure (d band center); in turn these properties will be correlated to the Pt thin film ORR activity. The substrate termination will induce different surface termination in the Pt thin films and, hence, the influence of the latter on the ORR activity will be also of interest.
The main goal of the present work is to gain further understanding of the relation between Pt interatomic distance and surface termination and its electrocatalytic activity towards ORR. These properties can be studied on thin films. Pulsed laser deposition represents a suitable technique for fabricating thin Pt films for electrocatalysis of varying crystalline orientation and strain.
A novel class of Pt-based catalyst will be developed by fabricating epitaxial thin films of Pt on single crystal strontium titanate (STO) using pulsed laser deposition. Due to the structural similarities (e.g. lattice parameters) of the substrates and Pt, a heteroepitaxial growth of a strained, thin Pt film is expected. The effect of the strain induced by the substrate will be investigated in terms of Pt interatomic distance and electronic structure (d band center); in turn these properties will be correlated to the Pt thin film ORR activity. The substrate termination will induce different surface termination in the Pt thin films and, hence, the influence of the latter on the ORR activity will be also of interest.
- V. Jalan and E. J. Taylor, J. Electrochem. Soc 130, 2299-2302 (1983)
- J.K.Norskov, J.Rossmeisl, A.Logadottir and L.Lindqvist, J. Phys. Chem. B 108, 17886-17892 (2004).
- S.Mukerjee, J.Appl.Electrochemistry 20, 537-548 (1990)
- N.M.Markovic, T.J.Schmidt, V.Stamenkovic, P.N.Ross, Fuel Cells 1, (2001)

![Schematic energy diagrams of photocatalytic water splitting by one-step and two-step photoexcitation systems. C.B., conduction band; V.B., valence band; Eg, band gap; NHE, normal hydrogen electrode. Figure has been adopted from Ref [1].](/sites/default/files/styles/primer_full_image_xxl/public/import/lmx-interfaces/ThinFilmMaterialsCatalysisEN/EnergyDiagramWaterSplitting.JPG.webp?itok=XBHhqKHP)