Suchen
How important is hydrogen for the energy transition?
Assessments by PSI energy expert Thomas J. Schmidt
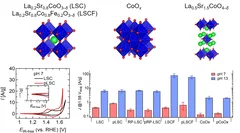
Improving the oxygen evolution reaction activity of Co-based oxides by phosphate functionalization
Our findings disclose that P-functionalization successfully enhances the oxygen evolution reaction (OER) activity of different cobalt-based catalysts (namely, La0.2Sr0.8CoO3–δ, La0.2Sr0.8Co0.8Fe0.2O3–δ, and CoOx) at near-neutral pHs and that both phosphate ion assistance in the OER mechanism and catalyst Co oxidation state can play a role in the enhanced OER activity.
Energie et climat
Aperçu de sujets
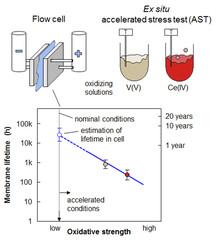
Membrane Lifetime Estimation in a Vanadium Redox Flow Battery using an Accelerated Stress Test
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
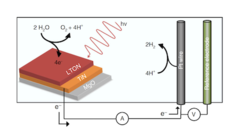
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
Zooming in on water splitting
Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
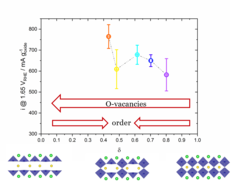
Correlation between Oxygen Vacancies and Oxygen Evolution Reaction Activity for a Model Electrode: PrBaCo2O5+δ
The role of the oxygen stoichiometry of perovskite catalysts in the oxygen evolution reaction (OER) is systematically studied in the PrBaCo2O5+δ family. The reduced number of physical/chemical variables combined with in-depth characterizations such as neutron diffraction, O K-edge X-ray absorption spectroscopy(XAS), electron energy loss spectroscopy (EELS), magnetization and scanning transmission electron microscopy (STEM) studies, helps investigating the complex correlation between OER activity and a single perovskite property, such as the oxygen content. Larger amount of oxygen vacancies appears to facilitate the OER, possibly contributing to the mechanism involving the oxidation of lattice oxygen, i.e., the lattice oxygen evolution reaction (LOER). Furthermore, not only the number of vacancies but also their local arrangement in the perovskite lattice influences the OER activity, with a clear drop for the more stable, ordered stoichiometry.
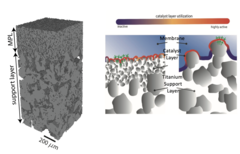
Hierarchically Structured Porous Transport Layers for Polymer Electrolyte Water Electrolysis
The high operational and capital costs of polymer electrolyte water electrolysis technology originate from limited catalyst utilization and the use of thick membrane electrolytes. PSI researchers have developed novel multi-layer porous transport materials, which provide superior electrochemical performance in comparison to conventional single-layer structures.
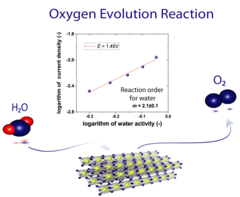
Understanding of the Oxygen Evolution Reaction Kinetics in Acidic Environment
The high operational expenditure of polymer electrolyte water electrolysis (PEWE) technology, dominated by kinetic losses from the sluggish oxygen evolution reaction (OER), inhibits large-scale market penetration. PSI researchers have developed a novel methodology to access underlying reaction mechanism of the OER. For the first time the reaction order for water has been determined. Advanced benchmarking of catalysts in technical environment also supports the development of novel, highly efficient catalyst materials.