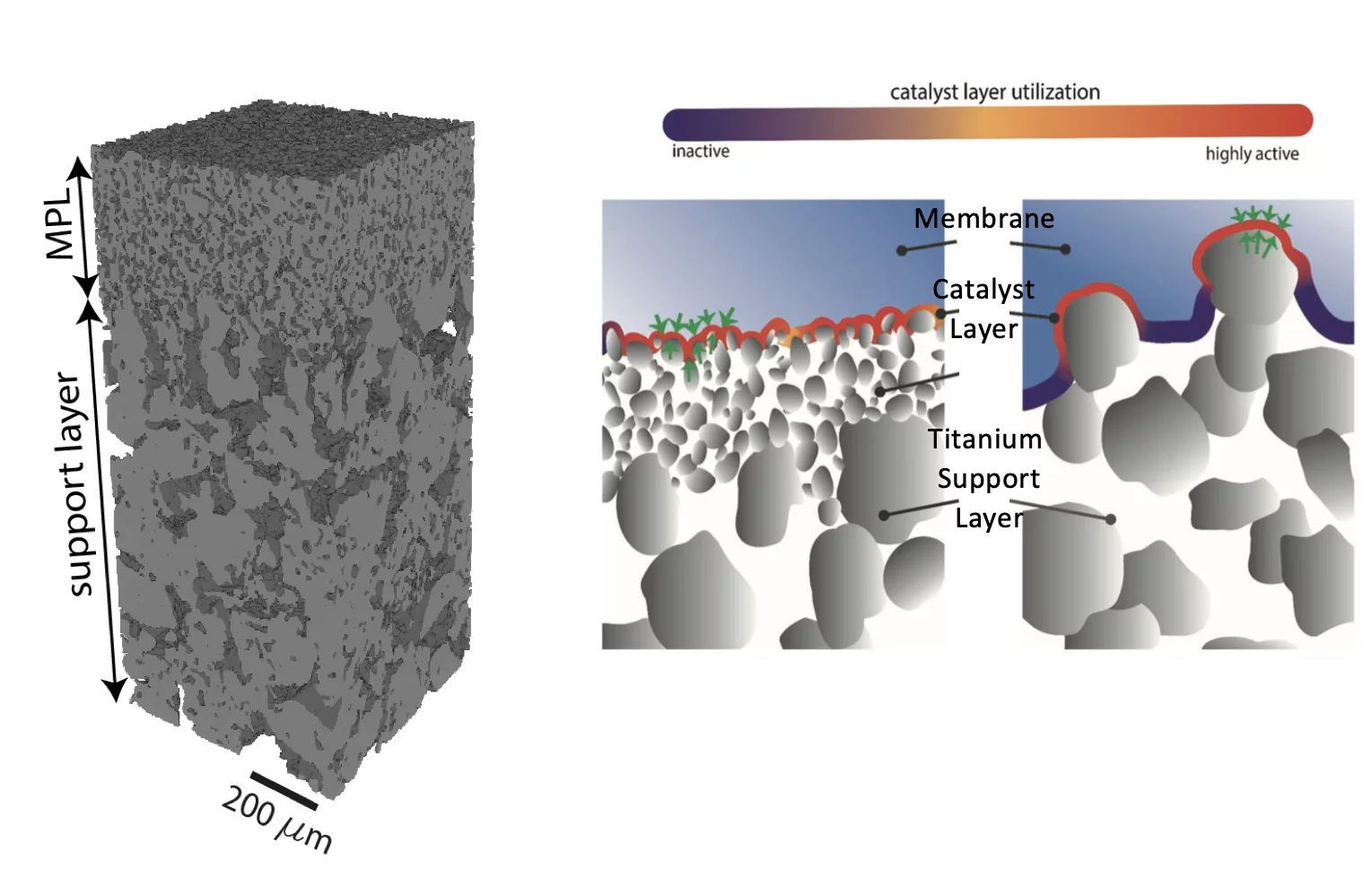
The high operational and capital costs of polymer electrolyte water electrolysis technology originate from limited catalyst utilization and the use of thick membrane electrolytes. PSI researchers have developed novel multi-layer porous transport materials, which provide superior electrochemical performance in comparison to conventional single-layer structures.
Conventionally, in polymer electrolyte water electrolyzers, thick membrane electrolytes are used which leads to high operational cost because of high power consumption and the catalyst utilization is limited leading to increased capital cost because of the required high noble metal catalyst loadings. This is due to the coarse surface structure of the state-of-the-art titanium porous transport layer materials used. Therefore, a series of materials with three different microporous layers (MPLs) with advanced interface properties are fabricated and characterized. It is shown that these sintered multilayer structures, made from economically viable titanium powders, have improved interface properties with low surface roughness, as characterized by X-ray laboratory and synchrotron-based tomographic microscopy. The transport layer materials provide superior electrochemical performance in comparison to conventional single-layer structures, with up to three times higher catalyst layer utilization and a ≈60 mV decrease in (anodic) mass transport overpotential at 2 A cm−2. The MPLs combine preferential surface properties with high open porosity and low tortuosity of sinter materials, enabling for the first time the use of thin membranes, in combination with anodic titanium transport layers. The fundamental mechanism of the MPL effect is elucidated and shown to be based on a homogeneous contact pressure distribution, resulting in high catalyst utilization and low mass transport losses.
Contact
Dr. Felix N. Büchi
Head Fuel Cell Systems and Diagnostics Group
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 24 10
E-mail: felix.buechi@psi.ch
Original Publication
Hierarchically Structured Porous Transport Layers for Polymer Electrolyte Water Electrolysis
Tobias Schuler, Joseph M. Ciccone, Bernd Krentscher, Federica Marone, Christian Peter, Thomas J. Schmidt, and Felix N. Büchi
Adv. Energy Mater. 10, 1903216 (2020)
DOI: 10.1002/aenm.201903216
Acknowledgement to Funding Agency
Swiss Federal Office of Energy (SFOE, Grant No. SI/501331-01) for funding of this project and Umicore (Belgium) for providing catalyst material for this study.