Our motto: A place where diversity lives, and sustainable energy is what it gives
Research Activities
The Electrocatalysis and Interfaces Group was established at PSI in 2012 and is now under the joint leadership of Prof. Thomas J. Schmidt and PD Dr. Emiliana Fabbri.
Our research activities focus on understanding the mechanisms of various electrochemical energy conversion reactions at solid-liquid interfaces. We aim to apply this fundamental understanding to the design of novel electrocatalysts for the oxygen reduction reaction (ORR), oxygen evolution reaction (OER), hydrogen evolution/oxidation reaction (HER/HOR), CO2 reduction reaction (CORR), and urea electrosynthesis.
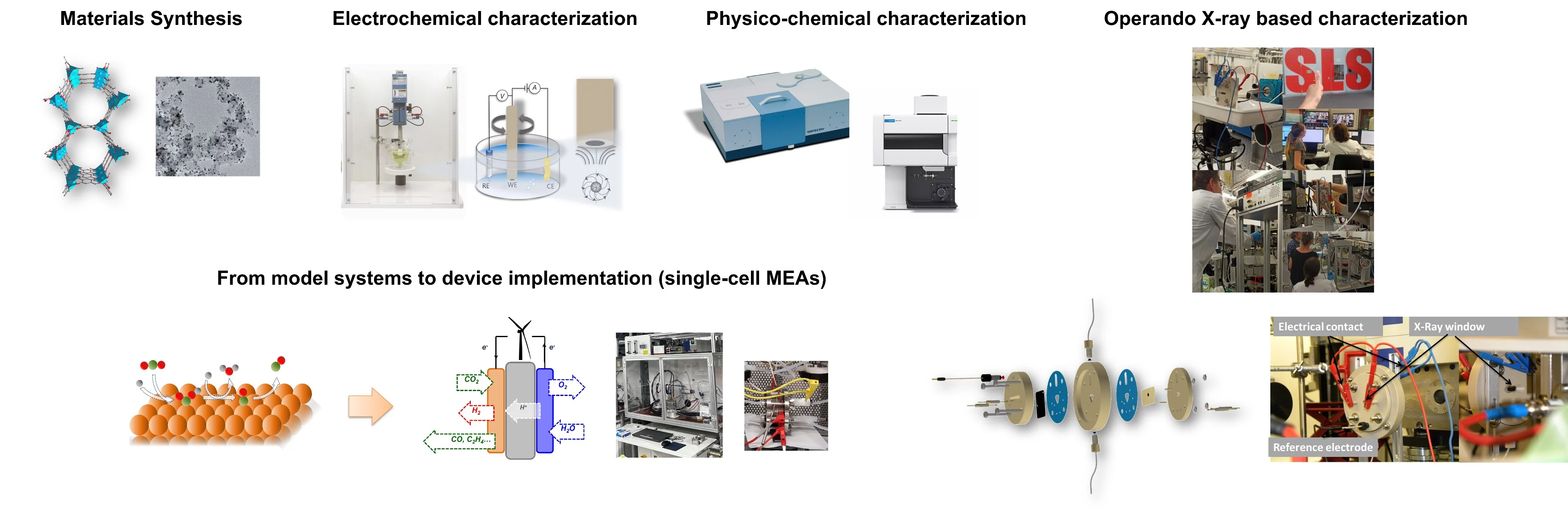
To optimize the rate, selectivity, energy efficiency and stability of these electrochemical reactions, appropriate catalysts must be designed and optimized. Our research studies include the synthesis of nanostructured materials, precise electrochemical characterization, and investigation of the physicochemical properties of the electrocatalysts under operating conditions using advanced X-ray-based operando spectroscopy. The most promising electrocatalysts developed by our group are often tested at the single cell level in (co)electrolyzers or fuel cells.
Our Vision
Through collaborative understanding, discovery, and creation, we will empower a future where science tackles society's greatest challenges and inspires a diverse generation of innovators.
Understand, Discover, and Create
- We strongly support the role of science in the development of society. Our research goals push the boundaries of science to create new values to address the major challenges facing our society: sustainable energy, climate changes and circular economy.
- We transfer fundamental discoveries into practical technologies.
- We enable our scientific excellence through collaboration.
Inspire and Educate
- We aim to provide a stimulating environment and an open learning space.
- An inclusive working environment and safe working conditions are essential for the professional development of our group members.
- We strive for: gender equality, high diversity (cultural, educational, gender), constructive team interaction, methodical & logical thinking, self-motivated team members and a supportive team environment.
Our Mission
- Pushing the boundaries of science through cutting-edge research on electrochemical energy conversion reactions, we strive for a sustainable society based on a clean and environmentally friendly energy system.
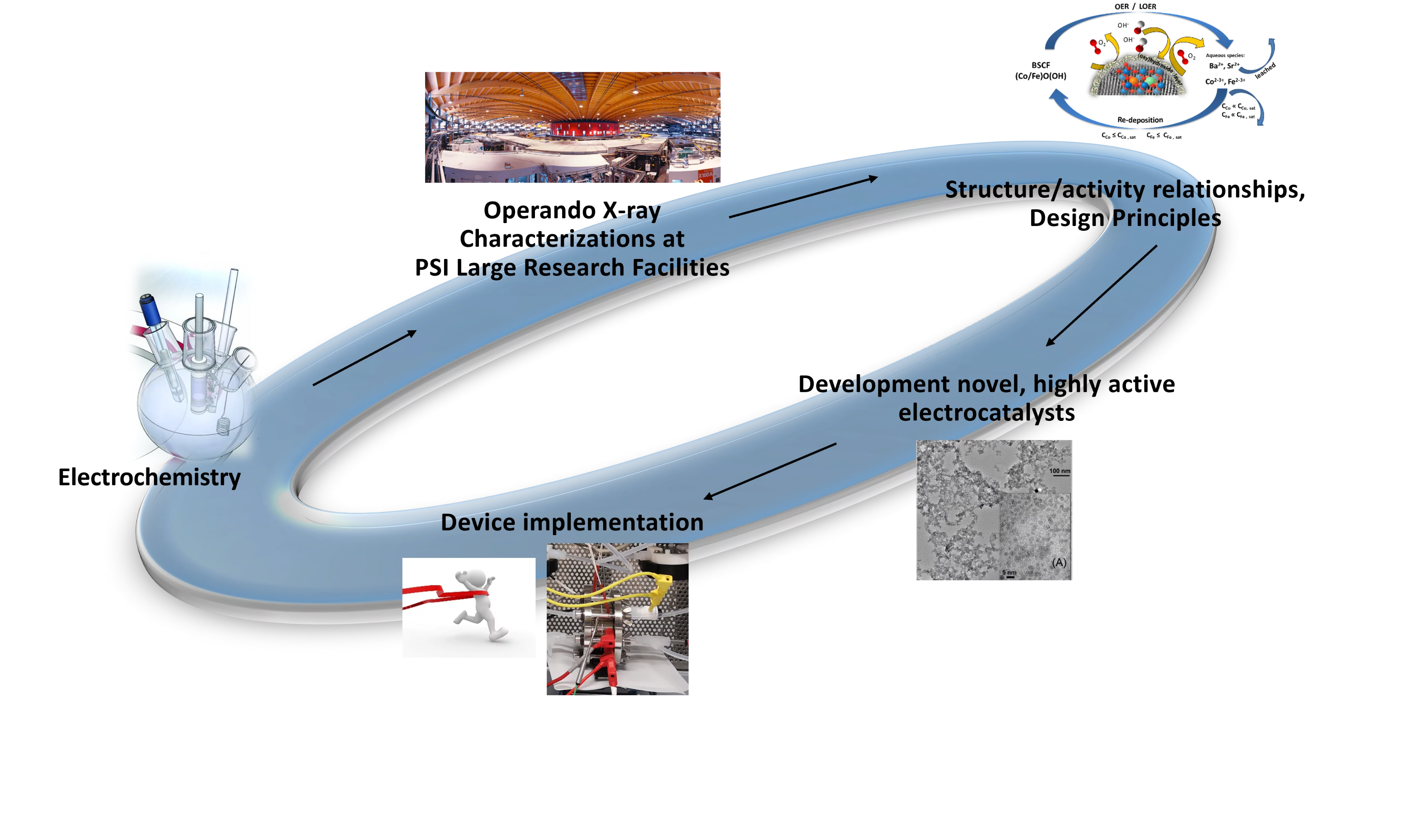
- Our goal is to understand reaction mechanisms, catalyst property/activity relationships, and design principles for various electrochemical reactions in order to develop better performing, longer lasting electrocatalysts.
- We aim to bridge the gap between fundamental science and application, ranging from model systems to device implementation (single cell MEAs).
Our Strategy
- Focus on technologies of future relevance.
- Material concept to device implementation TRL 1 – 2.
- Exploiting the possibilities of PSI’s large facilities.
Research Team
- Emiliana Fabbri, co-group leader
- Thomas J. Schmidt, co-group leader
- Marija Zoric, scientist
- Javier Quilez Bermejo, scientist
- Kenneth Crossley, PhD
- Liliane Dongmo Medonbou, postdoc
- Tim Welmers, PhD
- Juliana Bruneli Falqueto, postdoc
- Chopra Cheshta, guest PhD student
- Erica Clinton, PhD student
- Mateusz Wojtas, PhD student
- Jan Gotheer, semester student
- Paul Olli, semester student
- Natori Soichiro, visiting PhD student
- Mairis Iesalnieks, visiting PhD student
Group Events and News
Publications
For a complete list of publications visit https://orcid.org/0000-0002-8627-6926 (E. Fabbri) and https://orcid.org/0000-0002-1636-367X (T.J. Schmidt).
-
Aegerter D, Fabbri E, Novotny Z, Borlaf M, Yüzbasi NS, Comini N, et al.
Evaluation of dip-and-pull ambient pressure X-ray photoelectron spectroscopy for investigating oxygen evolution reaction electrocatalysts
ACS Applied Energy Materials. 2025; 8(19): 14554-14567. https://doi.org/10.1021/acsaem.5c02252
DORA PSI -
Beall CE, Fabbri E, Falqueto JB, Siegrist S, Huang J, Hales N, et al.
Composite bifunctional electrocatalyst for the oxygen reduction and evolution reactions
ACS Materials Au. 2025; 5(5): 798-808. https://doi.org/10.1021/acsmaterialsau.5c00034
DORA PSI -
Crossley K, Schmidt TJ, Fabbri E
Five key concepts linking vacancies, structure, and oxygen evolution reaction activity in cobalt-based electrocatalysts
Chemical Communications. 2025; 61(62): 11529-11537. https://doi.org/10.1039/d5cc02438b
DORA PSI -
Dörenkamp T, Zaccarelli A, Büchi FN, Schmidt TJ, Eller J
Guided water percolation in 3D-printed gas diffusion layers for polymer electrolyte fuel cells
ACS Applied Materials and Interfaces. 2025; 17(16): 23959 (13 pp.). https://doi.org/10.1021/acsami.5c00770
DORA PSI -
Hales N, Huang J, Sjølin BH, Garcia-Padilla A, Borca CN, Huthwelker T, et al.
Confining surface oxygen redox in double perovskites for enhanced oxygen evolution reaction activity and stability
Advanced Energy Materials. 2025; 15(25): 2404560 (14 pp.). https://doi.org/10.1002/aenm.202404560
DORA PSI -
Huang J, Zhang Z, Spezzati C, Clark AH, Hales N, Genz NS, et al.
Directly synthesized cobalt oxyhydroxide as an oxygen evolution catalyst in proton exchange membrane water electrolyzers
Nature Communications. 2025; 16(1): 7518 (14 pp.). https://doi.org/10.1038/s41467-025-62744-4
DORA PSI -
Huang J, Clark AH, Falqueto JB, Clinton ED, Crossley K, Schmidt TJ, et al.
Tracking the dynamic and interactive metal oxidation changes in CoFe, CoNi, and NiFe bimetallic hydroxides for electrocatalytic oxygen evolution
Advanced Functional Materials. 2025: e17223 (11 pp.). https://doi.org/10.1002/adfm.202517223
DORA PSI -
Huang J, Clinton ED, Crossley K, Falqueto JB, Schmidt TJ, Fabbri E
Uncovering the catalyst/electrolyte interfacial process by frequency dispersion of capacitance
Journal of Energy Chemistry. 2025; 108: 199-209. https://doi.org/10.1016/j.jechem.2025.04.024
DORA PSI -
Kwen J, Schmidt TJ, Herranz J
Impact of cathode components' configuration on the performance of forward-bias bipolar membrane CO2-electrolyzers
ACS Applied Energy Materials. 2025; 8(7): 4152-4165. https://doi.org/10.1021/acsaem.4c02883
DORA PSI -
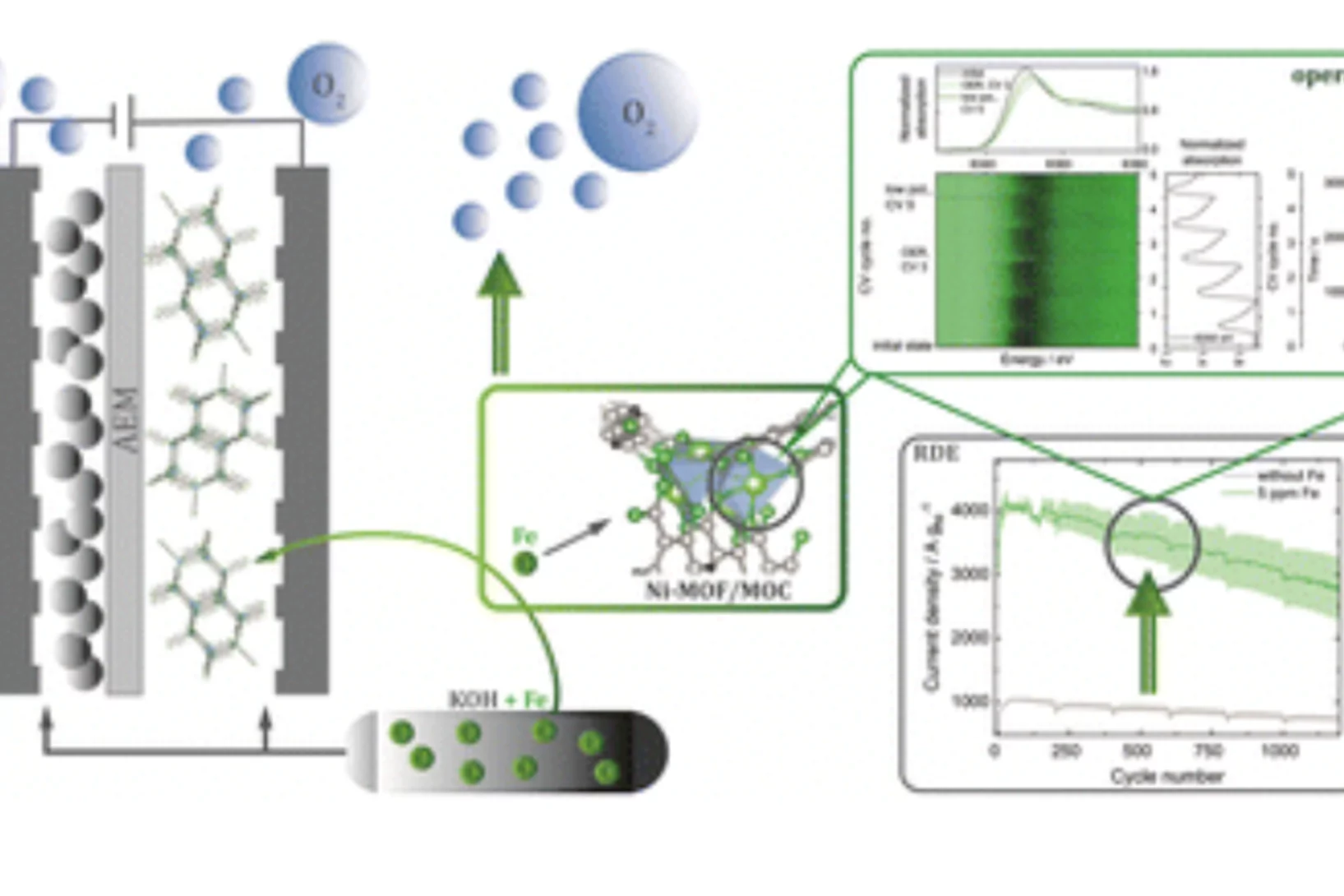
Linke J, Rohrbach T, Clark AH, Andrzejewski M, Casati NPM, Buchauer FL, et al.
From operando investigations to implementation of Ni-MOF-74 oxygen evolution electrocatalysts
Advanced Energy Materials. 2025: 2501401 (11 pp.). https://doi.org/10.1002/aenm.202501401
DORA PSI -
Linke J, Rohrbach T, Clark AH, Borca C, Huthwelker T, Buchauer FL, et al.
The role of Fe incorporation into Ni-MOF-74 derived oxygen evolution electrocatalysts for anion exchange membrane water electrolysis
EES Catalysis. 2025; 3: 505-514. https://doi.org/10.1039/d4ey00250d
DORA PSI -
Winzely M, Clark AH, Balalta D, Chauhan P, Leidinger PM, Fikry M, et al.
Monitoring the activation of a AuCu aerogel CO2-reduction electrocatalyst via operando XAS
Langmuir. 2025; 41: 11026-11036. https://doi.org/10.1021/acs.langmuir.5c00662
DORA PSI -
Wojtas M, Zorić MR, Fabbri E, Schmidt TJ
Quantification of urea in electrocatalytic systems
ChemElectroChem. 2025. https://doi.org/10.1002/celc.202500302
DORA PSI -
Aegerter D, Fabbri E, Borlaf M, Yüzbasi NS, Diklić N, Clark AH, et al.
Delving into Fe-content effects on surface reconstruction of Ba0.50Sr0.50Co1−xFexO3−δ for the oxygen evolution reaction
Journal of Materials Chemistry A. 2024; 12(9): 5156-5169. https://doi.org/10.1039/d3ta06156f
DORA PSI -
Beall CE, Fabbri E, Clark AH, Meier V, Yüzbasi NS, Graule T, et al.
Designing bifunctional perovskite catalysts for the oxygen reduction and evolution reactions
EES Catalysis. 2024; 2(5): 1152-1163. https://doi.org/10.1039/d4ey00084f
DORA PSI -
Beall CE, Fabbri E, Clark AH, Meier V, Yüzbasi NS, Sjølin BH, et al.
Time-resolved oxidation state changes are key to elucidating the bifunctionality of perovskite catalysts for oxygen evolution and reduction
Energy and Environmental Materials. 2024; 7(5): e12737 (9 pp.). https://doi.org/10.1002/eem2.12737
DORA PSI -
Chauhan P, Georgi M, Herranz J, Müller G, Diercks JS, Eychmüller A, et al.
Impact of surface composition changes on the CO2-reduction performance of Au-Cu aerogels
Langmuir. 2024; 40: 12288-12300. https://doi.org/10.1021/acs.langmuir.4c01511
DORA PSI -
Clark AH, Schmidt TJ, Fabbri E
Best practices for operando hard X-ray absorption spectroscopy
Nature Sustainability. 2024; 7: 688-691. https://doi.org/10.1038/s41893-024-01322-w
DORA PSI -
Duburg JC, Chen B, Holdcroft S, Schmidt TJ, Gubler L
Design of polybenzimidazolium membranes for use in vanadium redox flow batteries
Journal of Materials Chemistry A. 2024; 12(11): 6387-6398. https://doi.org/10.1039/d3ta07212f
DORA PSI -
Dörenkamp T, Büchi FN, Schmidt TJ, Eller J
Exploring chances and limitations of high resolution 3D-printing for guided water percolation in gas diffusion layers of polymer electrolyte fuel cells
InterPore Journal. 2024; 1(3): IPJ271124 (13 pp.). https://doi.org/10.69631/ipj.v1i3nr43
DORA PSI -
Dörenkamp T, Sabharwal M, Marone F, Büchi FN, Schmidt TJ, Eller J
Investigation of dynamic water cluster and droplet interactions in polymer electrolyte fuel cells using operando X-ray tomographic microscopy
Journal of the Electrochemical Society. 2024; 171(9): 094505 (9 pp.). https://doi.org/10.1149/1945-7111/ad749f
DORA PSI -
Fabbri E, Schmidt TJ
Operando X-ray absorption spectroscopy as a powerful tool for uncovering property-activity relationships for oxygen evolution transition metal oxide catalysts
Chimia. 2024; 78(5): 320-325. https://doi.org/10.2533/chimia.2024.320
DORA PSI -
Falqueto JB, Hales N, Schimidt TJ, Fabbri E
Recent advances in nickel-based perovskite oxides for the electrocatalytic oxygen evolution reaction in alkaline electrolytes
ACS Materials Letters. 2024; 6(12): 5227-5241. https://doi.org/10.1021/acsmaterialslett.4c01471
DORA PSI -
Fikry M, García-Padilla Á, Herranz J, Khavlyuk P, Eychmüller A, Schmidt TJ
A comprehensive analysis of the overpotential losses in polymer electrolyte fuel cells
ACS Catalysis. 2024; 14(3): 1903-1913. https://doi.org/10.1021/acscatal.3c04797
DORA PSI -
Fikry M, Weiß N, Bozzetti M, Ünsal S, Georgi M, Khavlyuk P, et al.
Up-scaled preparation of Pt-Ni aerogel catalyst layers for polymer electrolyte fuel cell cathodes
ACS Applied Energy Materials. 2024; 7(3): 896-905. https://doi.org/10.1021/acsaem.3c01930
DORA PSI -
Hampson E, Duburg JC, Casella J, Schmidt TJ, Gubler L
A simple approach to balancing conductivity and capacity fade in vanadium redox flow batteries by the tunable pretreatment of polybenzimidazole membranes
Chemical Engineering Journal. 2024; 485: 149930 (11 pp.). https://doi.org/10.1016/j.cej.2024.149930
DORA PSI -
Heinritz A, Leidinger P, Buhk B, Herranz J, Schmidt TJ
A high-potential trapped state upon H2-starvation of a platinum electrode in aqueous electrolyte
Journal of the Electrochemical Society. 2024; 171(1): 014503 (3 pp.). https://doi.org/10.1149/1945-7111/ad170c
DORA PSI -
Heinritz A, Binninger T, Herranz J, Rodriguez P, Schmidt TJ
Hydrogen oxidation and evolution reaction on platinum in alkaline electrolyte: fixed reference vs overpotential and the effect of H2 concentration
Journal of the Electrochemical Society. 2024; 171(11): 114511 (11 pp.). https://doi.org/10.1149/1945-7111/ad8eff
DORA PSI -
Huang J, Hales N, Clark AH, Yüzbasi NS, Borca CN, Huthwelker T, et al.
Operando tracking the interactions between CoOx and CeO2 during oxygen evolution reaction
Advanced Energy Materials. 2024; 14(11): 2303529 (10 pp.). https://doi.org/10.1002/aenm.202303529
DORA PSI -
Huang J, Clark AH, Hales N, Borca CN, Huthwelker T, Skoupy R, et al.
Spectroscopic investigations of complex electronic interactions by elemental doping and material compositing of cobalt oxide for enhanced oxygen evolution reaction activity
Advanced Functional Materials. 2024; 34(44): 2405384 (9 pp.). https://doi.org/10.1002/adfm.202405384
DORA PSI -
Huang J, Borca CN, Huthwelker T, Yüzbasi NS, Baster D, El Kazzi M, et al.
Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment
Nature Communications. 2024; 15(1): 3067 (9 pp.). https://doi.org/10.1038/s41467-024-47409-y
DORA PSI -
Liu W, Lee J, Schmidt TJ, Boillat P
Quantitative observation of water phase transition in gas diffusion layers utilizing advanced in situ differential scanning calorimetry
Journal of Power Sources. 2024; 602: 234268 (10 pp.). https://doi.org/10.1016/j.jpowsour.2024.234268
DORA PSI -
Marelli E, Lyu J, Morin M, Leménager M, Shang T, Yüzbasi NS, et al.
Cobalt-free layered perovskites RBaCuFeO5+δ (R = 4f lanthanide) as electrocatalysts for the oxygen evolution reaction
EES Catalysis. 2024; 2(1): 335-350. https://doi.org/10.1039/D3EY00142C
DORA PSI -
Saveleva VA, Herranz J, Schmidt TJ
Quantifying the kinetic parameters of fuel cell reactions
In: Alonso-Vante N, Di Noto V, eds. Electrocatalysis for membrane fuel cells. Methods, modeling, and applications. Weinheim: WILEY-VCH GmbH; 2024:111-147. https://doi.org/10.1002/9783527830572.ch4
DORA PSI -
Schuller A, Schmidt TJ, Eller J
Non-invasive measurement of impedance spectra distribution in polymer electrolyte fuel cells
Journal of the Electrochemical Society. 2024; 171(3): 034517 (7 pp.). https://doi.org/10.1149/1945-7111/ad2ba8
DORA PSI -
Winter E, Briccola M, Schmidt TJ, Trabesinger S
Enabling LiNO3 in carbonate electrolytes by flame-retardant electrolyte additive as a cosolvent for enhanced performance of lithium metal batteries
Applied Research. 2024; 3(1): e202200096 (11 pp.). https://doi.org/10.1002/appl.202200096
DORA PSI -
Winzely M, Clark AH, Diercks JS, Safonova O, Rüttimann P, Leidinger PM, et al.
Electrochemical cell for operando grazing-incidence X-ray absorption spectroscopic studies of low-loaded electrodes
Analytical Chemistry. 2024; 96(52): 20454-20464. https://doi.org/10.1021/acs.analchem.4c04233
DORA PSI -
Zoric MR, Fabbri E, Herranz J, Schmidt TJ
In situ and operando spectroscopic techniques for electrochemical energy storage and conversion applications
Journal of Physical Chemistry C. 2024; 128(45): 19055-19070. https://doi.org/10.1021/acs.jpcc.4c05526
DORA PSI -
Aegerter D, Fabbri E, Yüzbasi NS, Diklić N, Clark AH, Nachtegaal M, et al.
Co1-xFexOy oxygen evolution nanocatalysts: on the way to resolve (electro)chemically triggered surface-bulk discrepancy
ACS Catalysis. 2023; 13: 15899-15909. https://doi.org/10.1021/acscatal.3c04138
DORA PSI -
Beall CE, Fabbri E, Clark AH, Yüzbasi NS, Graule T, Schmidt TJ
Influence of carbon on the dynamic changes in Co oxidation state of Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite catalyst during the oxygen reduction and evolution reactions
EcoMat. 2023; 5(7): e12353 (9 pp.). https://doi.org/10.1002/eom2.12353
DORA PSI -
Berger A, Chen Y-C, Gatzemeier J, Schmidt TJ, Büchi FN, Gasteiger HA
Analysis of the MPL/GDL interface: impact of MPL intrusion into the GDL substrate
Journal of the Electrochemical Society. 2023; 170(9): 094509 (20 pp.). https://doi.org/10.1149/1945-7111/acfa26
DORA PSI -
Chauhan P, Herranz J, Winzely M, Georgi M, Khavlyuk P, Eychmüller A, et al.
Interfacial pH and product selectivity measurements during CO2 reduction on a rotating ring-disk electrode
Journal of Physical Chemistry C. 2023; 127(33): 16453-16463. https://doi.org/10.1021/acs.jpcc.3c04233
DORA PSI -
Chen Y-C, Dörenkamp T, Csoklich C, Berger A, Marone F, Eller J, et al.
On the water transport mechanism through the microporous layers of operando polymer electrolyte fuel cells probed directly by X-ray tomographic microscopy
Energy Advances. 2023; 2(9): 1447-1463. https://doi.org/10.1039/d3ya00189j
DORA PSI -
Diercks JS, Herranz J, Ebner K, Diklić N, Georgi M, Chauhan P, et al.
Spectroscopy vs. electrochemistry: catalyst layer thickness effects on operando/in situ measurements
Angewandte Chemie International Edition. 2023; 62(16): e202216633 (7 pp.). https://doi.org/10.1002/anie.202216633
DORA PSI -
Diklić N, Beard A, Herranz J, Heinritz A, Cen T, Garbe S, et al.
Breaking down the performance losses in O2-evolution stability tests of IrO2-based electrocatalysts
Journal of the Electrochemical Society. 2023; 170(7): 074503 (13 pp.). https://doi.org/10.1149/1945-7111/ace741
DORA PSI -
Diklić N, Clark AH, Herranz J, Aegerter D, Diercks JS, Beard A, et al.
Surface Ir+5 formation as a universal prerequisite for O2 evolution on Ir oxides
ACS Catalysis. 2023; 13(16): 11069-11079. https://doi.org/10.1021/acscatal.3c01448
DORA PSI -
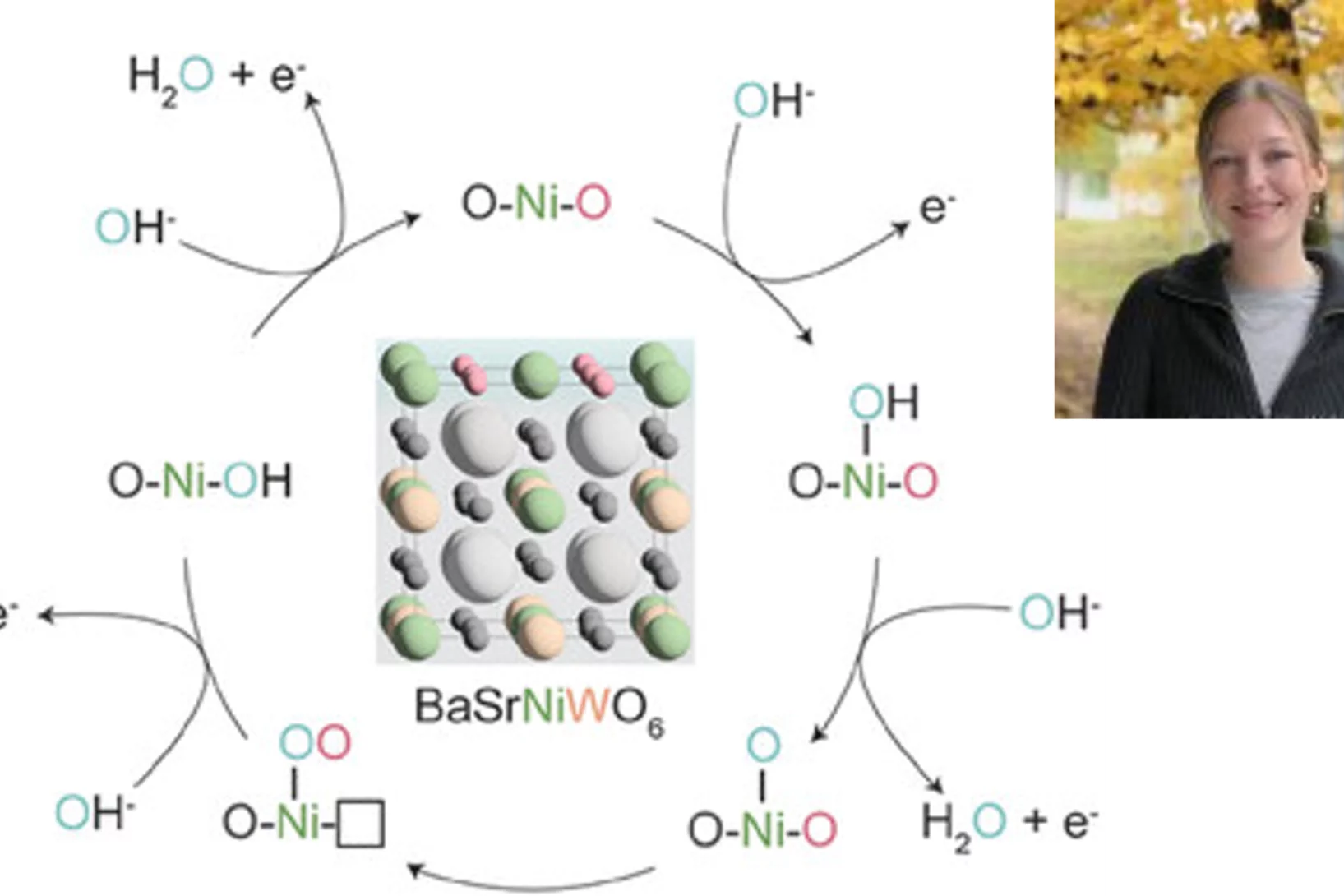
Hales N, Schmidt TJ, Fabbri E
Reversible and irreversible transformations of Ni-based electrocatalysts during the oxygen evolution reaction
Current Opinion in Electrochemistry. 2023; 38: 101231 (9 pp.). https://doi.org/10.1016/j.coelec.2023.101231
DORA PSI -
Liu W, Lee J, Manzi-Orezzoli V, Ntalis M, Schmidt TJ, Boillat P
Effects of hydrophobicity treatment of gas diffusion layers on ice crystallization in polymer electrolyte fuel cells
ACS Applied Materials and Interfaces. 2023; 15(14): 17779-17790. https://doi.org/10.1021/acsami.2c22155
DORA PSI -
Striednig M, Mularczyk A, Liu W, Scheuble D, Cochet M, Boillat P, et al.
Evaporative cooling for polymer electrolyte fuel cells - an operando analysis at technical single cell level
Journal of Power Sources. 2023; 556: 232419 (10 pp.). https://doi.org/10.1016/j.jpowsour.2022.232419
DORA PSI -
Wang C, Herranz J, Hübner R, Schmidt TJ, Eychmüller A
Element distributions in bimetallic aerogels
Accounts of Chemical Research. 2023; 56(3): 237-247. https://doi.org/10.1021/acs.accounts.2c00491
DORA PSI -
Winter E, Schmidt TJ, Trabesinger S
Potentiostatic lithium plating as a fast method for electrolyte evaluation in lithium metal batteries
Electrochimica Acta. 2023; 439: 141547 (13 pp.). https://doi.org/10.1016/j.electacta.2022.141547
DORA PSI -
Yoshimune W, Falqueto JB, Clark AH, Yüzbasi NS, Graule T, Baster D, et al.
The role of phosphate functionalization on the oxygen evolution reaction activity of cobalt‐based oxides at different pH values
Small Structures. 2023; 4(12): 2300106 (10 pp.). https://doi.org/10.1002/sstr.202300106
DORA PSI -
de Wild T, Wurm J, Becker P, Günther D, Nauser T, Schmidt TJ, et al.
A nature-inspired antioxidant strategy based on porphyrin for aromatic hydrocarbon containing fuel cell membranes**
ChemSusChem. 2023; 16(21): e202300775 (13 pp.). https://doi.org/10.1002/cssc.202300775
DORA PSI -
de Wild T, Nemeth T, Becker P, Günther D, Nauser T, Schmidt TJ, et al.
Repair of aromatic hydrocarbon-based membranes tested under accelerated fuel cell conditions
Journal of Power Sources. 2023; 560: 232525 (13 pp.). https://doi.org/10.1016/j.jpowsour.2022.232525
DORA PSI -
Ünsal S, Bozzetti M, Chen YC, Girod R, Berger A, Diercks JS, et al.
Catalyst aggregate size effect on the mass transport properties of non-noble metal catalyst layers for PEMFC cathodes
Journal of the Electrochemical Society. 2023; 170(7): 074502 (12 pp.). https://doi.org/10.1149/1945-7111/ace289
DORA PSI -
Ünsal S, Girod R, Appel C, Karpov D, Mermoux M, Maillard F, et al.
Decoupling the contributions of different instability mechanisms to the PEMFC performance decay of non-noble metal O2-reduction catalysts
Journal of the American Chemical Society. 2023; 145(14): 7845-7858. https://doi.org/10.1021/jacs.2c12751
DORA PSI -
Ünsal S, Schmidt TJ, Herranz J
Effect of aggregate size and film quality on the electrochemical properties of non-noble metal catalysts in rotating ring disk electrode measurements
Electrochimica Acta. 2023; 445: 142024 (9 pp.). https://doi.org/10.1016/j.electacta.2023.142024
DORA PSI -
Boucly A, Artiglia L, Fabbri E, Palagin D, Aegerter D, Pergolesi D, et al.
Direct evidence of cobalt oxyhydroxide formation on a La0.2Sr0.8CoO3 perovskite water splitting catalyst
Journal of Materials Chemistry A. 2022; 10(5): 2434-2444. https://doi.org/10.1039/D1TA04957G
DORA PSI -
Chauhan P, Hiekel K, Diercks JS, Herranz J, Saveleva VA, Khavlyuk P, et al.
Electrochemical surface area wuantification, CO2 reduction performance, and stability studies of unsupported three-dimensional Au aerogels versus carbon-supported Au nanoparticles
ACS Materials Au. 2022; 2(3): 278-292. https://doi.org/10.1021/acsmaterialsau.1c00067
DORA PSI -
Chen Y-C, Karageorgiou C, Eller J, Schmidt TJ, Büchi FN
Determination of the porosity and its heterogeneity of fuel cell microporous layers by X-ray tomographic microscopy
Journal of Power Sources. 2022; 539: 231612 (14 pp.). https://doi.org/10.1016/j.jpowsour.2022.231612
DORA PSI -
Csoklich C, Sabharwal M, Schmidt TJ, Büchi FN
Does the thermal conductivity of gas diffusion layer matter in polymer electrolyte fuel cells?
Journal of Power Sources. 2022; 540: 231539 (11 pp.). https://doi.org/10.1016/j.jpowsour.2022.231539
DORA PSI -
Csoklich C, Schmidt TJ, Büchi FN
High performance gas diffusion layers with added deterministic structures
Energy and Environmental Science. 2022; 15(3): 1293-1306. https://doi.org/10.1039/d1ee03246a
DORA PSI -
Diercks JS, Georgi M, Herranz J, Diklić N, Chauhan P, Clark AH, et al.
CO2 electroreduction on unsupported PdPt aerogels: effects of alloying and surface composition on product selectivity
ACS Applied Energy Materials. 2022; 5(7): 8460-8471. https://doi.org/10.1021/acsaem.2c00987
DORA PSI -
Diercks JS, Herranz J, Georgi M, Diklić N, Chauhan P, Ebner K, et al.
Interplay between surface-adsorbed CO and bulk Pd hydride under CO2-electroreduction conditions
ACS Catalysis. 2022; 12(17): 10727-10741. https://doi.org/10.1021/acscatal.2c02660
DORA PSI -
Diklić N, Clark AH, Herranz J, Diercks JS, Aegerter D, Nachtegaal M, et al.
Potential pitfalls in the operando XAS study of oxygen evolution electrocatalysts
ACS Energy Letters. 2022; 7(5): 1735-1740. https://doi.org/10.1021/acsenergylett.2c00727
DORA PSI -
Ebner K, Clark AH, Saveleva VA, Smolentsev G, Chen J, Ni L, et al.
Time-resolved potential-induced changes in Fe/N/C-catalysts studied by in situ modulation excitation X-ray absorption spectroscopy
Advanced Energy Materials. 2022; 12(14): 2103699 (14 pp.). https://doi.org/10.1002/aenm.202103699
DORA PSI -
Mourouga G, Chery D, Baudrin E, Randriamahazaka H, Schmidt TJ, Schumacher JO
Estimation of activity coefficients for aqueous organic redox flow batteries: theoretical basis and equations
iScience. 2022; 25(9): 104901 (26 pp.). https://doi.org/10.1016/j.isci.2022.104901
DORA PSI -
Pribyl-Kranewitter B, Beard A, Gîjiu CL, Dinculescu D, Schmidt TJ
Influence of low-temperature electrolyser design on economic and environmental potential of CO and HCOOH production: a techno-economic assessment
Renewable and Sustainable Energy Reviews. 2022; 154: 111807 (18 pp.). https://doi.org/10.1016/j.rser.2021.111807
DORA PSI -
Schuller A, Schmidt TJ, Eller J
Finite element model based determination of local membrane conductivity of polymer electrolyte fuel cells
Journal of the Electrochemical Society. 2022; 169(4): 044525 (7 pp.). https://doi.org/10.1149/1945-7111/ac6390
DORA PSI -
Schuller A, Schmidt TJ, Eller J
Non-invasive measurement of humidity distribution in polymer electrolyte fuel cells (PEFCs): part I. In situ proof of concept
Journal of the Electrochemical Society. 2022; 169(7): 074504 (8 pp.). https://doi.org/10.1149/1945-7111/ac7a62
DORA PSI -
Schuller A, Schmidt TJ, Eller J
Noninvasive measurement of humidity distribution in polymer electrolyte fuel cells (PEFCs). Part II: operando analysis of a fuel cell stack
Journal of the Electrochemical Society. 2022; 169(12): 124512 (10 pp.). https://doi.org/10.1149/1945-7111/aca0e3
DORA PSI -
Striednig M, Cochet M, Boillat P, Schmidt TJ, Büchi FN
A model based investigation of evaporative cooling for polymer electrolyte fuel cells - stack level analysis
Journal of Power Sources. 2022; 517: 230706 (11 pp.). https://doi.org/10.1016/j.jpowsour.2021.230706
DORA PSI -
Striednig M, Schmidt TJ, Büchi FN
A model based investigation of evaporative cooling for polymer electrolyte fuel cells - system level analysis
Journal of Power Sources. 2022; 542: 231720 (11 pp.). https://doi.org/10.1016/j.jpowsour.2022.231720
DORA PSI -
Winter E, Schmidt TJ, Trabesinger S
Identifying pitfalls in lithium metal battery characterization
Batteries and Supercaps. 2022; 5(1): e202100145 (13 pp.). https://doi.org/10.1002/batt.202100145
DORA PSI -
Xu H, Bührer M, Marone F, Schmidt TJ, Büchi FN, Eller J
Effects of gas diffusion layer substrates on PEFC water management: part II. In situ liquid water removal via evaporation
Journal of the Electrochemical Society. 2022; 169(10): 104503 (13 pp.). https://doi.org/10.1149/1945-7111/ac94a2
DORA PSI -
Beall CE, Fabbri E, Schmidt TJ
Perovskite oxide based electrodes for the oxygen reduction and evolution reactions: the underlying mechanism
ACS Catalysis. 2021; 11(5): 3094-3114. https://doi.org/10.1021/acscatal.0c04473
DORA PSI -
Chen Y-C, Berger A, De Angelis S, Schuler T, Bozzetti M, Eller J, et al.
A method for spatial quantification of water in microporous layers of polymer electrolyte fuel cells by X-ray tomographic microscopy
ACS Applied Materials and Interfaces. 2021; 13(14): 16227-16237. https://doi.org/10.1021/acsami.0c22358
DORA PSI -
Csoklich C, Steim R, Marone F, Schmidt TJ, Büchi FN
Gas diffusion layers with deterministic structure for high performance polymer electrolyte fuel cells
ACS Applied Materials and Interfaces. 2021; 13(8): 9908-9918. https://doi.org/10.1021/acsami.0c20896
DORA PSI -
Csoklich C, Xu H, Marone F, Schmidt TJ, Büchi FN
Laser structured gas diffusion layers for improved water transport and fuel cell performance
ACS Applied Energy Materials. 2021; 4(11): 12808-12818. https://doi.org/10.1021/acsaem.1c02454
DORA PSI -
De Angelis S, Schuler T, Charalambous MA, Marone F, Schmidt TJ, Büchi FN
Unraveling two-phase transport in porous transport layer materials for polymer electrolyte water electrolysis
Journal of Materials Chemistry A. 2021; 9(38): 22102-22113. https://doi.org/10.1039/d1ta03379d
DORA PSI -
Diercks JS, Pribyl-Kranewitter B, Herranz J, Chauhan P, Faisnel A, Schmidt TJ
An online gas chromatography cell setup for accurate CO2-electroreduction product quantification
Journal of the Electrochemical Society. 2021; 168(6): 064504 (11 pp.). https://doi.org/10.1149/1945-7111/ac0363
DORA PSI -
Duburg JC, Azizi K, Primdahl S, Hjuler HA, Zanzola E, Schmidt TJ, et al.
Composite polybenzimidazole membrane with high capacity retention for vanadium redox flow batteries
Molecules. 2021; 26(6): 1679 (15 pp.). https://doi.org/10.3390/molecules26061679
DORA PSI -
Ebner K, Ni L, Saveleva VA, Le Monnier BP, Clark AH, Krumeich F, et al.
57Fe-enrichment effect on the composition and performance of Fe-based O2-reduction electrocatalysts
Physical Chemistry Chemical Physics. 2021; 23(15): 9147-9157. https://doi.org/10.1039/d1cp00707f
DORA PSI -
Garbe S, Samulesson E, Schmidt TJ, Gubler L
Comparison of Pt-doped membranes for gas crossover suppression in polymer electrolyte water electrolysis
Journal of the Electrochemical Society. 2021; 168(10): 104502 (8 pp.). https://doi.org/10.1149/1945-7111/ac2925
DORA PSI -
Garbe S, Futter J, Schmidt TJ, Gubler L
Insight into elevated temperature and thin membrane application for high efficiency in polymer electrolyte water electrolysis
Electrochimica Acta. 2021; 377: 138046 (12 pp.). https://doi.org/10.1016/j.electacta.2021.138046
DORA PSI -
Garbe S, Futter J, Agarwal A, Tarik M, Mularczyk AA, Schmidt TJ, et al.
Understanding degradation effects of elevated temperature operating conditions in polymer electrolyte water electrolyzers
Journal of the Electrochemical Society. 2021; 168(4): 044515 (13 pp.). https://doi.org/10.1149/1945-7111/abf4ae
DORA PSI -
Heinritz A, Binninger T, Patru A, Schmidt TJ
Asymmetric Butler-Volmer kinetics of the electrochemical Ce(III)/Ce(IV) redox couple on polycrystalline Au electrodes in sulfuric acid and the dissociation field effect
ACS Catalysis. 2021; 11: 8140-8154. https://doi.org/10.1021/acscatal.0c04587
DORA PSI -
Iwase K, Ebner K, Diercks JS, Saveleva VA, Ünsal S, Krumeich F, et al.
Effect of cobalt speciation and the graphitization of the carbon matrix on the CO2 electroreduction activity of Co/N-doped carbon materials
ACS Applied Materials and Interfaces. 2021; 13(13): 15122-15131. https://doi.org/10.1021/acsami.0c21920
DORA PSI -
Kim B-J, Fabbri E, Borlaf M, Abbott DF, Castelli IE, Nachtegaal M, et al.
Oxygen evolution reaction activity and underlying mechanism of perovskite electrocatalysts at different pH
Materials Advances. 2021; 2(1): 345-355. https://doi.org/10.1039/D0MA00661K
DORA PSI -
Linke J, Rohrbach T, Ranocchiari M, Schmidt TJ, Fabbri E
Enlightening the journey of metal-organic framework (derived) catalysts during the oxygen evolution reaction in alkaline media via operando X-ray absorption spectroscopy
Current Opinion in Electrochemistry. 2021; 30: 100845 (7 pp.). https://doi.org/10.1016/j.coelec.2021.100845
DORA PSI -
Marelli E, Gazquez J, Poghosyan E, Müller E, Gawryluk DJ, Pomjakushina E, et al.
Correlation between oxygen vacancies and oxygen evolution reaction activity for a model electrode: PrBaCo2O5+δ
Angewandte Chemie International Edition. 2021; 60(26): 14609-14619. https://doi.org/10.1002/anie.202103151
DORA PSI -
Mularczyk A, Lin Q, Niblett D, Vasile A, Blunt MJ, Niasar V, et al.
Operando liquid pressure determination in polymer electrolyte fuel cells
ACS Applied Materials and Interfaces. 2021; 13(29): 34003-34011. https://doi.org/10.1021/acsami.1c04560
DORA PSI -
Mularczyk A, Michalski A, Striednig M, Herrendörfer R, Schmidt TJ, Büchi FN, et al.
Mass transport limitations ofwater evaporation in polymer electrolyte fuel cell gas diffusion layers
Energies. 2021; 14(10): 2967 (21 pp.). https://doi.org/10.3390/en14102967
DORA PSI -
Pittkowski RK, Abbott DF, Nebel R, Divanis S, Fabbri E, Castelli IE, et al.
Synergistic effects in oxygen evolution activity of mixed iridium-ruthenium pyrochlores
Electrochimica Acta. 2021; 366: 137327 (11 pp.). https://doi.org/10.1016/j.electacta.2020.137327
DORA PSI -
Pribyl-Kranewitter B, Beard A, Schuler T, Diklić N, Schmidt TJ
Investigation and optimisation of operating conditions for low- temperature CO2 reduction to CO in a forward-bias bipolar- membrane electrolyser
Journal of the Electrochemical Society. 2021; 168(4): 043506 (14 pp.). https://doi.org/10.1149/1945-7111/abf063
DORA PSI -
Saveleva VA, Ebner K, Ni L, Smolentsev G, Klose D, Zitolo A, et al.
Potential‐induced spin changes in Fe/N/C electrocatalysts assessed by in situ X‐ray emission spectroscopy
Angewandte Chemie International Edition. 2021; 60: 11707-11712. https://doi.org/10.1002/anie.202016951
DORA PSI -
Xu H, Bührer M, Marone F, Schmidt TJ, Büchi FN, Eller J
Effects of gas diffusion layer substrates on PEFC water management: Part I. Operando liquid water saturation and gas diffusion properties
Journal of the Electrochemical Society. 2021; 168(7): 074505 (14 pp.). https://doi.org/10.1149/1945-7111/ac1035
DORA PSI -
Zlobinski M, Schuler T, Büchi FN, Schmidt TJ, Boillat P
Elucidation of fluid streamlining in multi-layered porous transport layers for polymer electrolyte water electrolyzers by operando neutron radiography
Journal of the Electrochemical Society. 2021; 168(1): 014505 (9 pp.). https://doi.org/10.1149/1945-7111/abcf19
DORA PSI -
de Wild T, Nemeth T, Nolte TM, Schmidt TJ, Nauser T, Gubler L
Possible repair mechanism for hydrocarbon-based-ionomers following damage by radical attack
Journal of the Electrochemical Society. 2021; 168(5): 054514 (12 pp.). https://doi.org/10.1149/1945-7111/abf9be
DORA PSI -
Aegerter D, Borlaf M, Fabbri E, Clark AH, Nachtegaal M, Graule T, et al.
Tuning the Co oxidation state in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by flame spray synthesis towards high oxygen evolution reaction activity
Catalysts. 2020; 10(9): 984 (16 pp.). https://doi.org/10.3390/catal10090984
DORA PSI -
Babic U, Tarik M, Schmidt TJ, Gubler L
Understanding the effects of material properties and operating conditions on component aging in polymer electrolyte water electrolyzers
Journal of Power Sources. 2020; 451: 227778 (8 pp.). https://doi.org/10.1016/j.jpowsour.2020.227778
DORA PSI -
Boucly A, Fabbri E, Artiglia L, Cheng X, Pergolesi D, Ammann M, et al.
Surface segregation acts as surface engineering for the oxygen evolution reaction on perovskite oxides in alkaline media
Chemistry of Materials. 2020; 32(12): 5256-5263. https://doi.org/10.1021/acs.chemmater.0c01396
DORA PSI -
Halter J, Bevilacqua N, Zeis R, Schmidt TJ, Büchi FN
The impact of the catalyst layer structure on phosphoric acid migration in HT-PEFC – An operando X-ray tomographic microscopy study
Journal of Electroanalytical Chemistry. 2020; 859: 113832 (7 pp.). https://doi.org/10.1016/j.jelechem.2020.113832
DORA PSI -
Herranz J, Pătru A, Fabbri E, Schmidt TJ
Co-electrolysis of CO2 and H2O: from electrode reactions to cell-level development
Current Opinion in Electrochemistry. 2020; 23: 89-95. https://doi.org/10.1016/j.coelec.2020.05.004
DORA PSI -
Manzi-Orezzoli V, Siegwart M, Scheuble D, Chen Y-C, Schmidt TJ, Boillat P
Impact of the microporous layer on gas diffusion layers with patterned wettability I: material design and characterization
Journal of the Electrochemical Society. 2020; 167(6): 064516 (11 pp.). https://doi.org/10.1149/1945-7111/ab828f
DORA PSI -
Manzi-Orezzoli V, Siegwart M, Scheuble D, Schmidt TJ, Boillat P
Impact of the microporous layer on gas diffusion layers with patterned wettability II: operando performance and water distribution analysis by neutron imaging
Journal of the Electrochemical Society. 2020; 167(6): 064521 (12 pp.). https://doi.org/10.1149/1945-7111/ab8290
DORA PSI -
Manzi-Orezzoli V, Siegwart M, Cochet M, Schmidt TJ, Boillat P
Improved water management for PEFC with interdigitated flow fields using modified gas diffusion layers
Journal of the Electrochemical Society. 2020; 167(5): 054503 (8 pp.). https://doi.org/10.1149/2.0062005JES
DORA PSI -
Mularczyk A, Lin Q, Blunt MJ, Lamibrac A, Marone F, Schmidt TJ, et al.
Droplet and percolation network interactions in a fuel cell gas diffusion layer
Journal of the Electrochemical Society. 2020; 167(8): 084506 (8 pp.). https://doi.org/10.1149/1945-7111/ab8c85
DORA PSI -
Novotny Z, Aegerter D, Comini N, Tobler B, Artiglia L, Maier U, et al.
Probing the solid-liquid interface with tender x rays: a new ambient-pressure x-ray photoelectron spectroscopy endstation at the Swiss Light Source
Review of Scientific Instruments. 2020; 91(2): 023103 (10 pp.). https://doi.org/10.1063/1.5128600
DORA PSI -
Schuler T, Ciccone JM, Krentscher B, Marone F, Peter C, Schmidt TJ, et al.
Hierarchically structured porous transport layers for polymer electrolyte water electrolysis
Advanced Energy Materials. 2020; 10(2): 1903216 (12 pp.). https://doi.org/10.1002/aenm.201903216
DORA PSI -
Schuler T, Kimura T, Schmidt TJ, Büchi FN
Towards a generic understanding of oxygen evolution reaction kinetics in polymer electrolyte water electrolysis
Energy and Environmental Science. 2020; 13(7): 2153-2166. https://doi.org/10.1039/d0ee00673d
DORA PSI -
Siegwart M, Manzi-Orezzoli V, Valsecchi J, Harti RP, Kagias M, Strobl M, et al.
Operando visualization of water distribution in gas diffusion media of PEFCs with an optimized neutron grating interferometer
Journal of the Electrochemical Society. 2020; 167(6): 064509 (14 pp.). https://doi.org/10.1149/1945-7111/ab7d92
DORA PSI -
Siegwart M, Huang F, Cochet M, Schmidt TJ, Zhang J, Boillat P
Spatially resolved analysis of freezing during isothermal PEFC cold starts with time-of-flight neutron imaging
Journal of the Electrochemical Society. 2020; 167(6): 064510 (18 pp.). https://doi.org/10.1149/1945-7111/ab7d91
DORA PSI -
Xu H, Bührer M, Marone F, Schmidt TJ, Büchi FN, Eller J
Optimal image denoising for in situ X-ray tomographic microscopy of liquid water in gas diffusion layers of polymer electrolyte fuel cells
Journal of the Electrochemical Society. 2020; 167(10): 104505 (10 pp.). https://doi.org/10.1149/1945-7111/ab9820
DORA PSI -
Zlobinski M, Babic U, Fikry M, Gubler L, Schmidt TJ, Boillat P
Dynamic neutron imaging and modeling of cationic impurities in polymer electrolyte water electrolyzer
Journal of the Electrochemical Society. 2020; 167(14): 144509 (10 pp.). https://doi.org/10.1149/1945-7111/abc83b
DORA PSI -
Zlobinski M, Schuler T, Büchi FN, Schmidt TJ, Boillat P
Transient and steady state two-phase flow in anodic porous transport layer of proton exchange membrane water electrolyzer
Journal of the Electrochemical Society. 2020; 167(8): 084509 (9 pp.). https://doi.org/10.1149/1945-7111/ab8c89
DORA PSI -
Abbott DF, Pittkowski RK, Macounová K, Nebel R, Marelli E, Fabbri E, et al.
Design and synthesis of Ir/Ru pyrochlore catalysts for the oxygen evolution reaction based on their bulk thermodynamic properties
ACS Applied Materials and Interfaces. 2019; 11(41): 37748-37760. https://doi.org/10.1021/acsami.9b13220
DORA PSI -
Babic U, Zlobinski M, Schmidt TJ, Boillat P, Gubler L
CO2-assisted regeneration of a polymer electrolyte water electrolyzer contaminated with metal ion impurities
Journal of the Electrochemical Society. 2019; 166(10): F610-F619. https://doi.org/10.1149/2.0851910jes
DORA PSI -
Babic U, Nilsson E, Pătru A, Schmidt TJ, Gubler L
Proton transport in catalyst layers of a polymer electrolyte water electrolyzer: effect of the anode catalyst loading
Journal of the Electrochemical Society. 2019; 166(4): F214-F220. https://doi.org/10.1149/2.0341904jes
DORA PSI -
Cheng X, Kim B-J, Fabbri E, Schmidt TJ
Co/Fe oxyhydroxides supported on perovskite oxides as oxygen evolution reaction catalyst systems
ACS Applied Materials and Interfaces. 2019; 11(38): 34787-34795. https://doi.org/10.1021/acsami.9b04456
DORA PSI -
Ebner K, Herranz J, Saveleva VA, Kim B-J, Henning S, Demicheli M, et al.
Fe-based O2-reduction catalysts synthesized using Na2CO3as a pore-inducing agent
ACS Applied Energy Materials. 2019; 2(2): 1469-1479. https://doi.org/10.1021/acsaem.8b02036
DORA PSI -
Erbach S, Pribyl B, Klages M, Spitthoff L, Borah K, Epple S, et al.
Influence of operating conditions on permeation of CO2 through the membrane in an automotive PEMFC system
International Journal of Hydrogen Energy. 2019; 44(25): 12760-12771. https://doi.org/10.1016/j.ijhydene.2018.10.033
DORA PSI -
Garbe S, Babic U, Nilsson E, Schmidt TJ, Gubler L
Communication—Pt-doped thin membranes for gas crossover suppression in polymer electrolyte water electrolysis
Journal of the Electrochemical Society. 2019; 166(13): F873-F875. https://doi.org/10.1149/2.0111913jes
DORA PSI -
Halter J, Gloor T, Amoroso B, Schmidt TJ, Büchi FN
Wetting properties of porous high temperature polymer electrolyte fuel cells materials with phosphoric acid
Physical Chemistry Chemical Physics. 2019; 21(24): 13126-13134. https://doi.org/10.1039/C9CP02149C
DORA PSI -
Kim B-J, Fabbri E, Castelli IE, Borlaf M, Graule T, Nachtegaal M, et al.
Fe-doping in double perovskite PrBaCo2(1-x)Fe2xO6-δ: insights into structural and electronic effects to enhance oxygen evolution catalyst stability
Catalysts. 2019; 9(3): 263 (17 pp.). https://doi.org/10.3390/catal9030263
DORA PSI -
Kim BJ, Fabbri E, Abbott DF, Cheng X, Clark AH, Nachtegaal M, et al.
Functional role of Fe-doping in Co-based perovskite oxide catalysts for oxygen evolution reaction
Journal of the American Chemical Society. 2019; 141(13): 5231-5240. https://doi.org/10.1021/jacs.8b12101
DORA PSI -
Manzi-Orezzoli V, Mularczyk A, Trtik P, Halter J, Eller J, Schmidt TJ, et al.
Coating distribution analysis on gas diffusion layers for polymer electrolyte fuel cells by neutron and X-ray high-resolution tomography
ACS Omega. 2019; 4(17): 17236-17243. https://doi.org/10.1021/acsomega.9b01763
DORA PSI -
Oldenburg FJ, Ouarga A, Schmidt TJ, Gubler L
Accelerated stress test method for the assessment of membrane lifetime in vanadium redox flow batteries
ACS Applied Materials and Interfaces. 2019; 11(51): 47917-47928. https://doi.org/10.1021/acsami.9b15736
DORA PSI -
Oldenburg FJ, Nilsson E, Schmidt TJ, Gubler L
Tackling capacity fading in vanadium redox flow batteries with amphoteric polybenzimidazole/nafion bilayer membranes
ChemSusChem. 2019; 12(12): 2620-2627. https://doi.org/10.1002/cssc.201900546
DORA PSI -
Permyakova AA, Herranz J, El Kazzi M, Diercks JS, Povia M, Mangani LR, et al.
On the oxidation state of Cu2O upon electrochemical CO2 reduction: an XPS study
ChemPhysChem. 2019; 20(22): 3120-3127. https://doi.org/10.1002/cphc.201900468
DORA PSI -
Povia M, Abbott DF, Herranz J, Heinritz A, Lebedev D, Kim B-J, et al.
Operando X-ray characterization of high surface area iridium oxides to decouple their activity losses for the oxygen evolution reaction
Energy and Environmental Science. 2019; 12(10): 3038-3052. https://doi.org/10.1039/C9EE01018A
DORA PSI -
Pătru A, Binninger T, Pribyl B, Schmidt TJ
Design principles of bipolar electrochemical co-electrolysis cells for efficient reduction of carbon dioxide from gas phase at low temperature
Journal of the Electrochemical Society. 2019; 166(2): F34-F43. https://doi.org/10.1149/2.1221816jes
DORA PSI -
Schuler T, Schmidt TJ, Büchi FN
Polymer electrolyte water electrolysis: correlating performance and porous transport layer structure: part II. Electrochemical performance analysis
Journal of the Electrochemical Society. 2019; 166(10): F555-F565. https://doi.org/10.1149/2.1241908jes
DORA PSI -
Schuler T, De Bruycker R, Schmidt TJ, Büchi FN
Polymer electrolyte water electrolysis: correlating porous transport layer structural properties and performance: part I. Tomographic analysis of morphology and topology
Journal of the Electrochemical Society. 2019; 166(4): F270-F281. https://doi.org/10.1149/2.0561904jes
DORA PSI -
Siegwart M, Woracek R, Márquez Damián JI, Tremsin AS, Manzi-Orezzoli V, Strobl M, et al.
Distinction between super-cooled water and ice with high duty cycle time-of-flight neutron imaging
Review of Scientific Instruments. 2019; 90(10): 103705 (15 pp.). https://doi.org/10.1063/1.5110288
DORA PSI -
Siegwart M, Harti RP, Manzi-Orezzoli V, Valsecchi J, Strobl M, Grünzweig C, et al.
Selective visualization of water in fuel cell gas diffusion layers with neutron dark-field imaging
Journal of the Electrochemical Society. 2019; 166(2): F149-F157. https://doi.org/10.1149/2.1011902jes
DORA PSI -
Abbott DF, Fabbri E, Borlaf M, Bozza F, Schäublin R, Nachtegaal M, et al.
Operando X-ray absorption investigations into the role of Fe in the electrochemical stability and oxygen evolution activity of Ni1−xFexOy nanoparticles
Journal of Materials Chemistry A. 2018; 6(47): 24534-24549. https://doi.org/10.1039/C8TA09336A
DORA PSI -
Babic U, Schmidt TJ, Gubler L
Communication - contribution of catalyst layer proton transport resistance to voltage loss in polymer electrolyte water electrolyzers
Journal of the Electrochemical Society. 2018; 165(15): J3016-J3018. https://doi.org/10.1149/2.0031815jes
DORA PSI -
Binninger T, Pribyl B, Pătru A, Ruettimann P, Bjelić S, Schmidt TJ
Multivariate calibration method for mass spectrometry of interfering gases such as mixtures of CO, N2, and CO2
Journal of Mass Spectrometry. 2018; 53(12): 1214-1221. https://doi.org/10.1002/jms.4299
DORA PSI -
Chattot R, Le Bacq O, Beermann V, Kühl S, Herranz J, Henning S, et al.
Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis
Nature Materials. 2018; 17(9): 827-833. https://doi.org/10.1038/s41563-018-0133-2
DORA PSI -
Cheng X, Fabbri E, Yamashita Y, Castelli IE, Kim B, Uchida M, et al.
Oxygen evolution reaction on perovskites: a multieffect descriptor study combining experimental and theoretical methods
ACS Catalysis. 2018; 8(10): 9567-9578. https://doi.org/10.1021/acscatal.8b02022
DORA PSI -
Fabbri E, Schmidt TJ
Oxygen evolution reaction - the enigma in water electrolysis
ACS Catalysis. 2018; 8(10): 9765-9774. https://doi.org/10.1021/acscatal.8b02712
DORA PSI -
Halter J, Marone F, Schmidt TJ, Büchi FN
Breaking through the cracks: on the mechanism of phosphoric acid migration in high temperature polymer electrolyte fuel cells
Journal of the Electrochemical Society. 2018; 165(14): F1176-F1183. https://doi.org/10.1149/2.0501814jes
DORA PSI -
Halter J, Thomas S, Kær SK, Schmidt TJ, Büchi FN
The influence of phosphoric acid migration on the performance of high temperature polymer electrolyte fuel cells
Journal of Power Sources. 2018; 399: 151-156. https://doi.org/10.1016/j.jpowsour.2018.07.090
DORA PSI -
Henning S, Shimizu R, Herranz J, Kühn L, Eychmüller A, Uchida M, et al.
Unsupported Pt3Ni aerogels as corrosion resistant PEFC anode catalysts under gross fuel starvation conditions
Journal of the Electrochemical Society. 2018; 165(6): F3001-F3006. https://doi.org/10.1149/2.0531802jes
DORA PSI -
Ishikawa H, Henning S, Herranz J, Eychmüller A, Uchida M, Schmidt TJ
Tomographic analysis and modeling of polymer electrolyte fuel cell unsupported catalyst layers
Journal of the Electrochemical Society. 2018; 165(2): F7-F16. https://doi.org/10.1149/2.0371802jes
DORA PSI -
Kim B-J, Cheng X, Abbott DF, Fabbri E, Bozza F, Graule T, et al.
Highly active nanoperovskite catalysts for oxygen evolution reaction: insights into activity and stability of Ba0.5Sr0.5Co0.8Fe0.2O2+δ and PrBaCO2O5+δ
Advanced Functional Materials. 2018; 28(45): 1804355 (10 pp.). https://doi.org/10.1002/adfm.201804355
DORA PSI -
Mohamed R, Binninger T, Kooyman PJ, Hoell A, Fabbri E, Patru A, et al.
Facile deposition of Pt nanoparticles on Sb-doped SnO2 support with outstanding active surface area for the oxygen reduction reaction
Catalysis Science and Technology. 2018; 8(10): 2672-2685. https://doi.org/10.1039/c7cy02591b
DORA PSI -
Nagy G, Sproll V, Gasser U, Schmidt TJ, Gubler L, Balog S
Scaling the graft length and graft density of irradiation-grafted copolymers
Macromolecular Chemistry and Physics. 2018; 219(21): 1800311 (7 pp.). https://doi.org/10.1002/macp.201800311
DORA PSI -
Oldenburg FJ, Bon M, Perego D, Polino D, Laino T, Gubler L, et al.
Revealing the role of phosphoric acid in all-vanadium redox flow batteries with DFT calculations and in situ analysis
Physical Chemistry Chemical Physics. 2018; 20(36): 23664-23673. https://doi.org/10.1039/c8cp04517h
DORA PSI -
Povia M, Herranz J, Binninger T, Nachtegaal M, Diaz A, Kohlbrecher J, et al.
Combining SAXS and XAS to study the operando degradation of carbon-supported Pt-nanoparticle fuel cell catalysts
ACS Catalysis. 2018; 8(8): 7000-7015. https://doi.org/10.1021/acscatal.8b01321
DORA PSI -
Rabis A, Binninger T, Fabbri E, Schmidt TJ
Impact of support physicochemical properties on the CO oxidation and the oxygen reduction reaction activity of Pt/SnO2 electrocatalysts
Journal of Physical Chemistry C. 2018; 122(9): 4739-4746. https://doi.org/10.1021/acs.jpcc.7b09976
DORA PSI -
Rabis A, Prokscha T, Fabbri E, Salman Z, Schmidt T, Suter A
Investigation of hydrogen-like muonium states in Nb-doped SnO2 films
In: Koda A, ed. Proceedings of the 14th international conference on muon spin rotation, relaxation and resonance (μSR2017). Vol. 21. JPS conference proceedings. Tokyo: Physical Society of Japan; 2018:011033 (6 pp.). https://doi.org/10.7566/JPSCP.21.011033
DORA PSI -
Sproll V, Schmidt TJ, Gubler L
Effect of glycidyl methacrylate (GMA) incorporation on water uptake and conductivity of proton exchange membranes
Radiation Physics and Chemistry. 2018; 144: 276-279. https://doi.org/10.1016/j.radphyschem.2017.08.025
DORA PSI -
Suermann M, Schmidt TJ, Büchi FN
Comparing the kinetic activation energy of the oxygen evolution and reduction reactions
Electrochimica Acta. 2018; 281: 466-471. https://doi.org/10.1016/j.electacta.2018.05.150
DORA PSI -
Taylor SM, Pătru A, Perego D, Fabbri E, Schmidt TJ
Influence of carbon material properties on activity and stability of the negative electrode in vanadium redox flow batteries: a model electrode study
ACS Applied Energy Materials. 2018; 1(3): 1166-1174. https://doi.org/10.1021/acsaem.7b00273
DORA PSI -
Abbott DF, Meier M, Meseck GR, Fabbri E, Seeger S, Schmidt TJ
Silicone nanofilament-supported mixed nickel-metal oxides for alkaline water electrolysis
Journal of the Electrochemical Society. 2017; 164(4): F203-F208. https://doi.org/10.1149/2.0201704jes
DORA PSI -
Babic U, Suermann M, Büchi FN, Gubler L, Schmidt TJ
Review—identifying critical gaps for polymer electrolyte water electrolysis development
Journal of the Electrochemical Society. 2017; 164(4): F387-F399. https://doi.org/10.1149/2.1441704jes
DORA PSI -
Binninger T, Schmidt TJ, Kramer D
Capacitive electronic metal-support interactions: outer surface charging of supported catalyst particles
Physical Review B. 2017; 96(16): 165405 (11 pp.). https://doi.org/10.1103/PhysRevB.96.165405
DORA PSI -
Binninger T, Mohamed R, Patru A, Waltar K, Gericke E, Tuaev X, et al.
Stabilization of Pt nanoparticles due to electrochemical transistor switching of oxide support conductivity
Chemistry of Materials. 2017; 29(7): 2831-2843. https://doi.org/10.1021/acs.chemmater.6b04851
DORA PSI -
Cai B, Henning S, Herranz J, Schmidt TJ, Eychmüller A
Nanostructuring noble metals as unsupported electrocatalysts for polymer electrolyte fuel cells
Advanced Energy Materials. 2017; 7(23): 1700548 (16 pp.). https://doi.org/10.1002/aenm.201700548
DORA PSI -
Cheng X, Fabbri E, Kim B, Nachtegaal M, Schmidt TJ
Effect of ball milling on the electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3 towards the oxygen evolution reaction
Journal of Materials Chemistry A. 2017; 5(25): 13130-13137. https://doi.org/10.1039/c7ta00794a
DORA PSI -
Fabbri E, Abbott DF, Nachtegaal M, Schmidt TJ
Operando X-ray absorption spectroscopy: a powerful tool toward water splitting catalyst development
Current Opinion in Electrochemistry. 2017; 5(1): 20-26. https://doi.org/10.1016/j.coelec.2017.08.009
DORA PSI -
Fabbri E, Rabis A, Chino Y, Uchida M, Schmidt TJ
Boosting Pt oxygen reduction reaction activity by tuning the tin oxide support
Electrochemistry Communications. 2017; 83: 90-95. https://doi.org/10.1016/j.elecom.2017.09.006
DORA PSI -
Fabbri E, Nachtegaal M, Binninger T, Cheng X, Kim B-J, Durst J, et al.
Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting
Nature Materials. 2017; 16(9): 925-931. https://doi.org/10.1038/nmat4938
DORA PSI -
Forner-Cuenca A, Manzi-Orezzoli V, Kristiansen PM, Gubler L, Schmidt TJ, Boillat P
Mask-assisted electron radiation grafting for localized through-volume modification of porous substrates: influence of electron energy on spatial resolution
Radiation Physics and Chemistry. 2017; 135: 133-141. https://doi.org/10.1016/j.radphyschem.2017.01.036
DORA PSI -
Henning S, Herranz J, Ishikawa H, Kim BJ, Abbott D, Kühn L, et al.
Durability of unsupported Pt-Ni aerogels in PEFC cathodes
Journal of the Electrochemical Society. 2017; 164(12): F1136-F1141. https://doi.org/10.1149/2.0131712jes
DORA PSI -
Henning S, Kühn L, Herranz J, Nachtegaal M, Hübner R, Werheid M, et al.
Effect of acid washing on the oxygen reduction reaction activity of Pt-Cu aerogel catalysts
Electrochimica Acta. 2017; 233: 210-217. https://doi.org/10.1016/j.electacta.2017.03.019
DORA PSI -
Henning S, Ishikawa H, Kühn L, Herranz J, Müller E, Eychmüller A, et al.
Unsupported Pt-Ni aerogels with enhanced high current performance and durability in fuel cell cathodes
Angewandte Chemie International Edition. 2017; 56(36): 10707-10710. https://doi.org/10.1002/anie.201704253
DORA PSI -
Karim W, Tschupp SA, Herranz J, Schmidt TJ, Ekinci Y, van Bokhoven JA
State-of-the-art nanofabrication in catalysis
Chimia. 2017; 71(4): 160-169. https://doi.org/10.2533/chimia.2017.160
DORA PSI -
Kim B-J, Abbott DF, Cheng X, Fabbri E, Nachtegaal M, Bozza F, et al.
Unraveling thermodynamics, stability, and oxygen evolution activity of strontium ruthenium perovskite oxide
ACS Catalysis. 2017; 7(5): 3245-3256. https://doi.org/10.1021/acscatal.6b03171
DORA PSI -
Lebedev D, Povia M, Waltar K, Abdala PM, Castelli IE, Fabbri E, et al.
Highly active and stable iridium pyrochlores for oxygen evolution reaction
Chemistry of Materials. 2017; 29(12): 5182-5191. https://doi.org/10.1021/acs.chemmater.7b00766
DORA PSI -
Nibel O, Rojek T, Schmidt TJ, Gubler L
Amphoteric ion-exchange membranes with significantly improved vanadium barrier properties for all-vanadium redox flow batteries
ChemSusChem. 2017; 10(13): 2767-2777. https://doi.org/10.1002/cssc.201700610
DORA PSI -
Nibel O, Taylor SM, Pǎtru A, Fabbri E, Gubler L, Schmidt TJ
Performance of different carbon electrode materials: insights into stability and degradation under real vanadium redox flow battery operating conditions
Journal of the Electrochemical Society. 2017; 164(7): A1608-A1615. https://doi.org/10.1149/2.1081707jes
DORA PSI -
Nibel O, Bon M, Agiorgousis ML, Laino T, Gubler L, Schmidt TJ
Unraveling the interaction mechanism between amidoxime groups and vanadium ions at various pH conditions
Journal of Physical Chemistry C. 2017; 121(12): 6436-6445. https://doi.org/10.1021/acs.jpcc.6b12540
DORA PSI -
Oakton E, Lebedev D, Povia M, Abbott DF, Fabbri E, Fedorov A, et al.
IrO2-TiO2: a high-surface-area, active, and stable electrocatalyst for the oxygen evolution reaction
ACS Catalysis. 2017; 7(4): 2346-2352. https://doi.org/10.1021/acscatal.6b03246
DORA PSI -
Oezaslan M, Herrmann A-K, Werheid M, Frenkel AI, Nachtegaal M, Dosche C, et al.
Structural analysis and electrochemical properties of bimetallic palladium–platinum aerogels prepared by a two-step gelation process
ChemCatChem. 2017; 9(5): 798-808. https://doi.org/10.1002/cctc.201600667
DORA PSI -
Oldenburg FJ, Schmidt TJ, Gubler L
Tackling capacity fading in vanadium flow batteries with amphoteric membranes
Journal of Power Sources. 2017; 368: 68-72. https://doi.org/10.1016/j.jpowsour.2017.09.051
DORA PSI -
Sproll V, Handl M, Hiesgen R, Friedrich KA, Schmidt TJ, Gubler L
Membrane architecture with ion-conducting channels through swift heavy ion induced graft copolymerization
Journal of Materials Chemistry A. 2017; 5(47): 24826-24835. https://doi.org/10.1039/c7ta07323b
DORA PSI -
Suermann M, Kiupel T, Schmidt TJ, Büchi FN
Electrochemical hydrogen compression: efficient pressurization concept derived from an energetic evaluation
Journal of the Electrochemical Society. 2017; 164(12): F1187-F1195. https://doi.org/10.1149/2.1361712jes
DORA PSI -
Suermann M, Pătru A, Schmidt TJ, Büchi FN
High pressure polymer electrolyte water electrolysis: test bench development and electrochemical analysis
International Journal of Hydrogen Energy. 2017; 42(17): 12076-12086. https://doi.org/10.1016/j.ijhydene.2017.01.224
DORA PSI -
Suermann M, Takanohashi K, Lamibrac A, Schmidt TJ, Büchi FN
Influence of operating conditions and material properties on the mass transport losses of polymer electrolyte water electrolysis
Journal of the Electrochemical Society. 2017; 164(9): F973-F980. https://doi.org/10.1149/2.13517109jes
DORA PSI -
Taylor SM, Pătru A, Fabbri E, Schmidt TJ
Influence of surface oxygen groups on V(II) oxidation reaction kinetics
Electrochemistry Communications. 2017; 75: 13-16. https://doi.org/10.1016/j.elecom.2016.12.003
DORA PSI -
Tschupp SA, Temmel SE, Salguero NP, Herranz J, Schmidt TJ
Numerical partitioning model for the Koutecký-Levich analysis of electrochemical flow cells with a combined Channel/Wall-Jet geometry
Journal of the Electrochemical Society. 2017; 164(11): E3448-E3456. https://doi.org/10.1149/2.0441711jes
DORA PSI -
Xu H, Bührer M, Marone F, Schmidt TJ, Büchi FN, Eller J
Fighting the noise: towards the limits of subsecond X-ray tomographic microscopy of PEFC
In: Jones DJ, Buechi F, Swider-Lyons KE, Pintauro PN, Uchida H, Schmidt TJ, et al., eds. Vol. 80. ECS transactions. Pennington, NJ: The Electrochemical Society; 2017:395-402. https://doi.org/10.1149/08008.0395ecst
DORA PSI -
Abbott DF, Lebedev D, Waltar K, Povia M, Nachtegaal M, Fabbri E, et al.
Iridium oxide for the oxygen evolution reaction: correlation between particle size, morphology, and the surface hydroxo layer from operando XAS
Chemistry of Materials. 2016; 28(18): 6591-6604. https://doi.org/10.1021/acs.chemmater.6b02625
DORA PSI -
Albert A, Lochner T, Schmidt TJ, Gubler L
Stability and degradation mechanisms of radiation-grafted polymer electrolyte membranes for water electrolysis
ACS Applied Materials and Interfaces. 2016; 8(24): 15297-15306. https://doi.org/10.1021/acsami.6b03050
DORA PSI -
Biesdorf J, Forner-Cuenca A, Siegwart M, Schmidt TJ, Boillat P
Statistical analysis of isothermal cold starts of PEFCs: impact of gas diffusion layer properties
Journal of the Electrochemical Society. 2016; 163(10): F1258-F1266. https://doi.org/10.1149/2.1071610jes
DORA PSI -
Binninger T, Fabbri E, Patru A, Garganourakis M, Han J, Abbott DF, et al.
Electrochemical flow-cell setup for in situ X-ray investigations. I. Cell for SAXS and XAS at synchrotron facilities
Journal of the Electrochemical Society. 2016; 163(10): H906-H912. https://doi.org/10.1149/2.0201610jes
DORA PSI -
Eberhardt SH, Marone F, Stampanoni M, Büchi FN, Schmidt TJ
Operando X-ray tomographic microscopy imaging of HT-PEFC: a comparative study of phosphoric acid electrolyte migration
Journal of the Electrochemical Society. 2016; 163(8): F842-F847. https://doi.org/10.1149/2.0801608jes
DORA PSI -
Engl T, Gubler L, Schmidt TJ
Fuel electrode carbon corrosion in high temperature polymer electrolyte fuel cells - crucial or irrelevant?
Energy Technology. 2016; 4(1): 65-74. https://doi.org/10.1002/ente.201500217
DORA PSI -
Forner-Cuenca A, Biesdorf J, Lamibrac A, Manzi-Orezzoli V, Büchi FN, Gubler L, et al.
Advanced water management in PEFCs: diffusion layers with patterned wettability. II. measurement of capillary pressure characteristic with neutron and synchrotron imaging
Journal of the Electrochemical Society. 2016; 163(9): F1038-F1048. https://doi.org/10.1149/2.0511609jes
DORA PSI -
Forner-Cuenca A, Biesdorf J, Manzi-Orezzoli V, Gubler L, Schmidt TJ, Boillat P
Advanced water management in PEFCs: diffusion layers with patterned wettability. III. operando characterization with neutron imaging
In: Savinell RF, ed. Vol. 163. Journal of the electrochemical society. sine loco: ECS; 2016:F1389-F1398. https://doi.org/10.1149/2.0891613jes
DORA PSI -
Forner-Cuenca A, Manzi-Orezzoli V, Biesdorf J, El Kazzi M, Streich D, Gubler L, et al.
Advanced water management in PEFCs: diffusion layers with patterned wettability: I. Synthetic routes, wettability tuning and thermal stability
Journal of the Electrochemical Society. 2016; 163(8): F788-F801. https://doi.org/10.1149/2.0271608jes
DORA PSI -
Henning S, Kühn L, Herranz J, Durst J, Binninger T, Nachtegaal M, et al.
Pt-Ni aerogels as unsupported electrocatalysts for the oxygen reduction reaction
Journal of the Electrochemical Society. 2016; 163(9): F998-F1003. https://doi.org/10.1149/2.0251609jes
DORA PSI -
Herranz J, Durst J, Fabbri E, Patru A, Cheng X, Permyakova AA, et al.
Interfacial effects on the catalysis of the hydrogen evolution, oxygen evolution and CO2-reduction reactions for (co-)electrolyzer development
Nano Energy. 2016; 29: 4-28. https://doi.org/10.1016/j.nanoen.2016.01.027
DORA PSI -
Kühn L, Herrmann A-K, Rutkowski B, Oezaslan M, Nachtegaal M, Klose M, et al.
Alloying behavior of self-assembled noble metal nanoparticles
Chemistry: A European Journal. 2016; 22(38): 13446-13450. https://doi.org/10.1002/chem.201602487
DORA PSI -
Nibel O, Schmidt TJ, Gubler L
Bifunctional ion-conducting polymer electrolyte for the vanadium redox flow battery with high selectivity
Journal of the Electrochemical Society. 2016; 163(13): A2562-A2570. https://doi.org/10.1149/2.0441613jes
DORA PSI -
Oakton E, Lebedev D, Fedorov A, Krumeich F, Tillier J, Sereda O, et al.
A simple one-pot Adams method route to conductive high surface area IrO2-TiO2 materials
New Journal of Chemistry. 2016; 40(2): 1834-1838. https://doi.org/10.1039/c5nj02400e
DORA PSI -
Oezaslan M, Liu W, Nachtegaal M, Frenkel AI, Rutkowski B, Werheid M, et al.
Homogeneity and elemental distribution in self-assembled bimetallic Pd-Pt aerogels prepared by a spontaneous one-step gelation process
Physical Chemistry Chemical Physics. 2016; 18(30): 20640-20650. https://doi.org/10.1039/c6cp03527b
DORA PSI
Former Group Members
| Nataša Diklić | 2018-2022 | Novamem |
| Justus Sebastian Diercks | 2018-2022 | Freudenberg |
| Bernhard Pribyl-Kranewitter | 2017-2021 | Kearney |
| Alexandra Patru | 2013-2020 | Sensirion |
| Kathrin Ebner | 2017-2020 | Bauhaus Luftfahrt |
| Viktoriia Saveleva | 2018-2020 | ESRF - European Synchrotron |
| Mauro Povia | 2015 - 2019 | Ecxelsus Structural Solutions |
| Daniel Abbott | 2015 - 2019 | ETH Zurich |
| Baejung (Joseph) Kim | 2015 - 2019 | Hyundai Mobis |
| Daniele Perego | 2017 - 2019 | |
| Anastasia A. Permyakova | 2014 - 2018 | ABB |
| Xi Cheng | 2014 - 2017 | PSI - Thin films and interfaces |
| Susan Taylor | 2014 - 2017 | RD Graphene |
| Tobias Binninger | 2012 - 2017 | NCCR Marvel |
| Simon Tschupp | 2013 - 2017 | Axetris |
| Sandra Temmel | 2012 - 2016 | Elring Klinger |
| Yohan Paratcha | 2014 - 2016 | |
| Annett Rabis | 2011 - 2015 | |
| Julien Durst | 2014 - 2015 | Air Liquide |
| Kay Waltar | 2013 - 2015 | ETH Zürich |
| Anja Habereder | 2013 - 2014 | |
| Mehtap Özaslan | 2012 - 2014 | Carl von Ossietzky Universität Oldenburg |
| Rüdiger Kötz | 1989 - 2014 | Elsevier |
| Annette Foelske-Schmitz | 2004 - 2013 | Technische Universität Wien |
| Paramaconi Rodriguez | 2011 - 2012 | University of Birmingham |
| Jorge Ferreira de Araújo | 2012 - 2013 | Technische Universität Berlin |