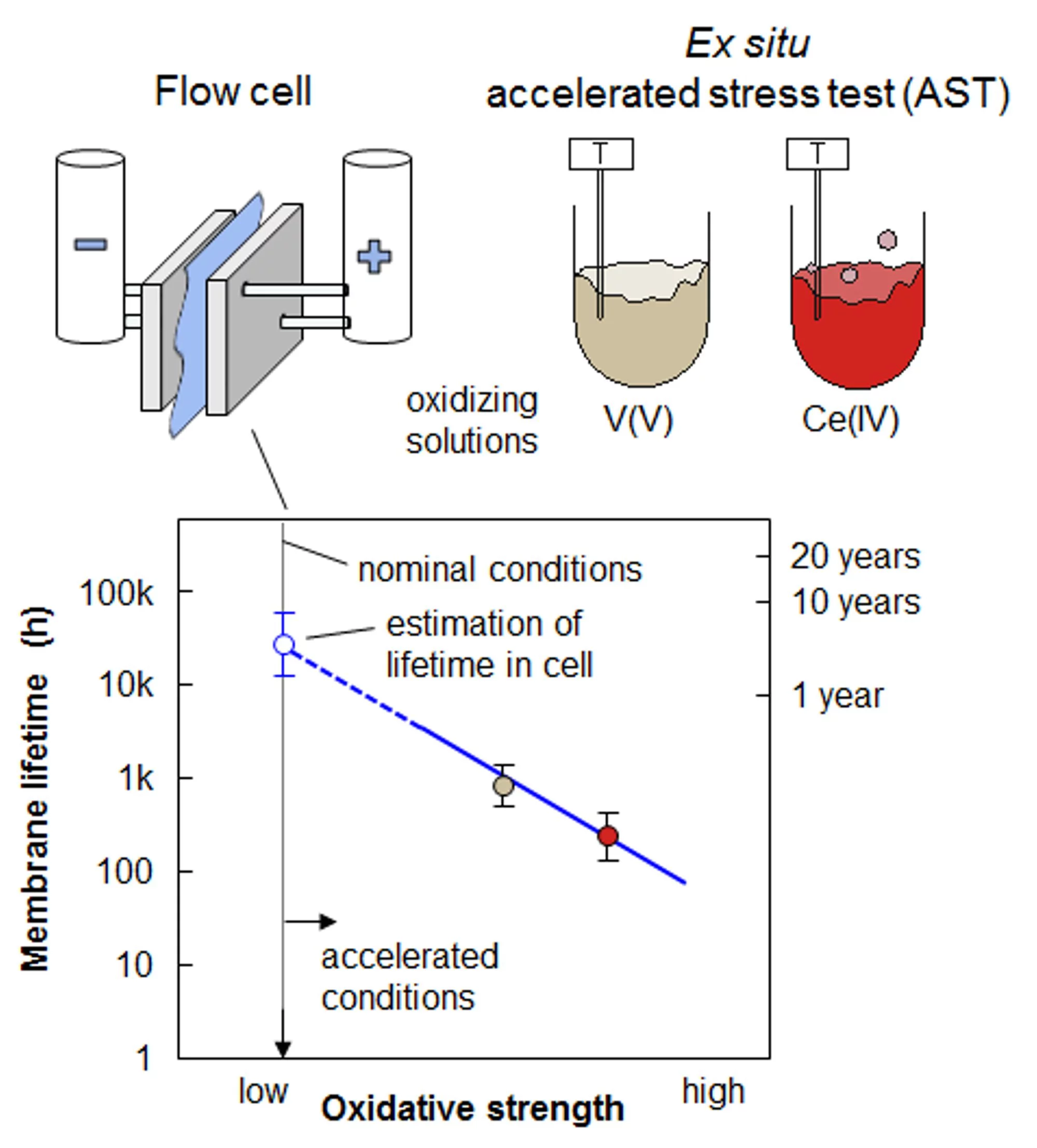
A vanadium redox flow battery (VRFB) is a grid-scale energy storage device. Its energy conversion unit consists of a cell stack that comprises ion-exchange membranes to separate positive and negative electrode. The projected lifetime of a VRFB is 20 years and 7’000 charge-discharge cycles. Lifetime tests of membranes under application relevant conditions are therefore impractical, and the development of an accelerated stress test (AST) to assess the chemical stability of membranes is crucial.
In the VRFB, vanadium-ions of different oxidation state are used to build a rechargeable electrochemical energy storage device (battery). Vanadium-ions of valence +II and +III are dissolved in an aqueous sulfuric acid in the negative electrolyte, and vanadium-ions of valence +IV and +V in the positive electrolyte. The vanadium +V, V(V), can cause oxidative membrane degradation. In our AST, membranes of various composition are immersed in a solution containing V(V) in 2 M sulfuric acid. The oxidative membrane degradation was accelerated by increasing the temperature of the solution and its oxidative strength. Therefore, we replaced V(V) by cerium(+IV), Ce(IV), which is a stronger oxidant than V(V). The degradation caused by Ce(IV) follows a similar reaction pathway but is faster by a factor of ~4 compared to V(V). The acceleration factor of the degradation reaction in Ce(IV) solution at 80°C is ~200 compared to the test in the VRFB single cell. The validity of this AST method was assessed by comparing tests in the cell with accelerated tests ex situ in the AST solution. Based on an in situ charge-discharge cell test over 1’500 cycles and post-test analysis of the state of health of the membrane, the lifetime of the membrane was projected to be 4’600 ± 900 h. The lifetime of the membrane estimated based on the ex situ AST was 3’300 h, which is within the limits of the projection. However, the uncertainty of the estimation is rather high (2’000 h), which is a result of limited number of statistically sound AST data points. Statistics of estimates are improved with a larger body of AST data.
For the application of the AST method to other membrane materials and polymer classes for vanadium and other flow battery types, the assumption of a consistent degradation mechanism and transferable composition-property relations to the conditions in the cell needs to be established and validated. Thus, the developed AST method can be a valuable tool for membrane and flow battery developers, together with other membrane characterization techniques, to screen and down-select candidate materials for development. It provides an “engineering test” or “index test” and can serve as a basis for predictive modeling of the membrane lifetime in VRFBs. With an acceleration factor of 200, tests over 500 h can be used to mimic membrane aging over 10 years under flow cell application conditions.
Contact
PD Dr. Lorenz Gubler
Paul Scherrer Institut
5232 Villligen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publication
Accelerated Stress Test Method for the Assessment of Membrane Lifetime in Vanadium Redox Flow Batteries
Fabio J. Oldenburg, Ayoub Ouarga, Thomas J. Schmidt, Lorenz Gubler
ACS Applied Materials & Interfaces
DOI: 10.1021/acsami.9b15736
Acknowledgement
The Swiss Federal Office of Energy (grant no. SI/501421-01) is kindly acknowledged for funding of the project. TJS. thanks Innosuisse and the Swiss Competence Center for Energy Research (SCCER) Heat and Electricity Storage. AO thanks the Materials Science and Nanoengineering Department (MSN) of Mohammed VI Polytechnic University (UM6P) for financially supporting the research stay at PSI.