Perovskite oxynitride materials can act as effective photocatalysts for water splitting driven by visible light. A combined neutron and x-ray study now provides unique insight into the underlying processes at the solid–liquid interface and highlights how solar-to-hydrogen conversion can be improved.
An appealing approach to generating sustainable energy is to harness sun light for splitting water, which in turn yields hydrogen fuel. For such a process to be practical, an efficient photocatalyst is needed that works in the visible-light range. A class of materials with promising characteristics in this respect are perovskite oxynitrides. Their potential for applications is currently limited, however, by a rapid loss of photoelectrochemical performance during operation. What causes that decline remains not fully understood. Writing in Nature Communications, Craig Lawley and colleagues in the Thin Films and Interfaces group of Prof. Thomas Lippert report now a new approach to characterising the processes at the oxynitride–water interface. By combining neutron reflectometry and x-ray absorption spectroscopy, both performed at PSI, they gained deep insight into how the surface of oxynitride thin films is modified during water-splitting operation — and they establish how the interface can be protected against degradation.
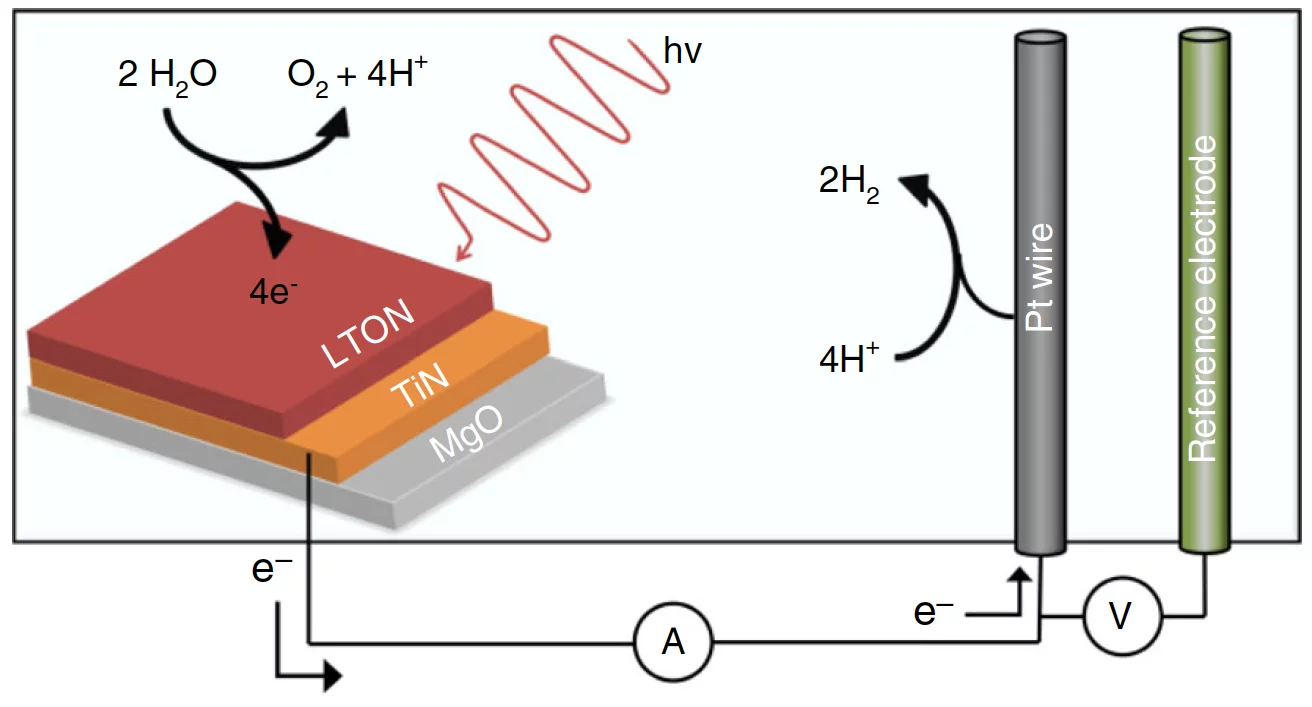
Photoelectrocatalysis is based on the light-induced generation of electron–hole pairs in a semiconductor. These charges then drive electrochemical reactions in the outside medium, such as water reduction and oxidation (see the figure). Perovskite oxynitrides are an attractive family of semiconductor materials for water splitting, as their band gap is narrow enough for excitation with visible light yet sufficiently wide to deliver the energy required to split water. In the past decade, substantial progress has been made in synthesizing oxynitride materials suitable for solar water splitting. The fundamental physiochemical processes at the oxynitride–liquid interface, and how they impede sustained operation, have received much less attention though.
Capturing water-splitting on film
The work of Lawley et al. fills that important gap and provides nanoscopic insight into how the surface of the oxynitride LaTiOxNy changes during water-splitting operation. Their data stand out for two reasons in particular. First, whereas in applications typically oxynitride powders are used, the team, led by PSI Senior Scientist Dr. Daniele Pergolesi, worked with thin films, which have a well-defined, atomically flat surface. This makes it possible to reliably distinguish surface and bulk contributions. Second, at PSI the researchers had access to two complementary surface-sensitive techniques, neutron reflectometry at SINQ and grazing-incidence x-ray absorption spectroscopy (GIXAS) at SLS. Both can be employed in situ, enabling the study of reactions in the presence of water; this is not possible, for example, for techniques that require vacuum operation.
A look beneath the surface
In the cell they developed (see the figure), the measured photocurrent dropped by some 20% within only three measurement cycles, the typical degree of decrease for such devices. Neutron reflectometry provided the data to model the density profile of the LaTiOxNy films. Changes due to water-splitting operation turned out to occur predominantly within the first 3 nm from the surface. A surprise came subsequently when Lawley and his co-workers looked at the element-specific spectroscopic data from the GIXAS experiments. These showed that the catalytically more active cations are not titanium, as previously assumed, but lanthanum. At the same time, a detailed analysis of the x-ray data revealed that the titanium octahedra of the perovskite structure become disordered within the 3-nm layer as a consequence of the water-splitting process. This modification, in turn, might explain the loss in performance.
But not all is lost. When the LaTiOxNy surfaces were decorated with a co-catalyst, IrO2, not only did the performance increase — an effect that had been observed before — but also the surface modifications were seen to be far less pronounced. Taken together, these findings provide new leads for the optimization of solar water-splitting photocatalysts. In the meanwhile, the PSI team is already busy building a new system for the next steps in exploring the fundamental mechanisms at play, in particular in operando x-ray measurements, which offer a glance at atomistic-scale changes as the photoelectrochemical processes happen.
The neutron reflectivity measurements were performed at the AMOR reflectometer at SINQ, and the XAS measurements at the SuperXAS beamline of the SLS. This work was carried out in collaboration with researchers at ETH Zurich, the University of Göttingen (Germany) and Kyushu University (Japan).
Contact
Dr. Daniele Pergolesi
Paul Scherrer Institute, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 4267, e-mail: daniele.pergolesi@psi.ch
Original Publication
1. Examining the surface evolution of LaTiOxNy an oxynitride solar water splitting photocatalyst
C. Lawley, M. Nachtegaal, J. Stahn, V. Roddatis, M. Döbeli, T. J. Schmidt, D. Pergolesi, T. Lippert
Nature Communications 11, 1728 (2020).
DOI: 10.1038/s41467-020-15519-y
Selected as an Editors’ Highlight on energy materials