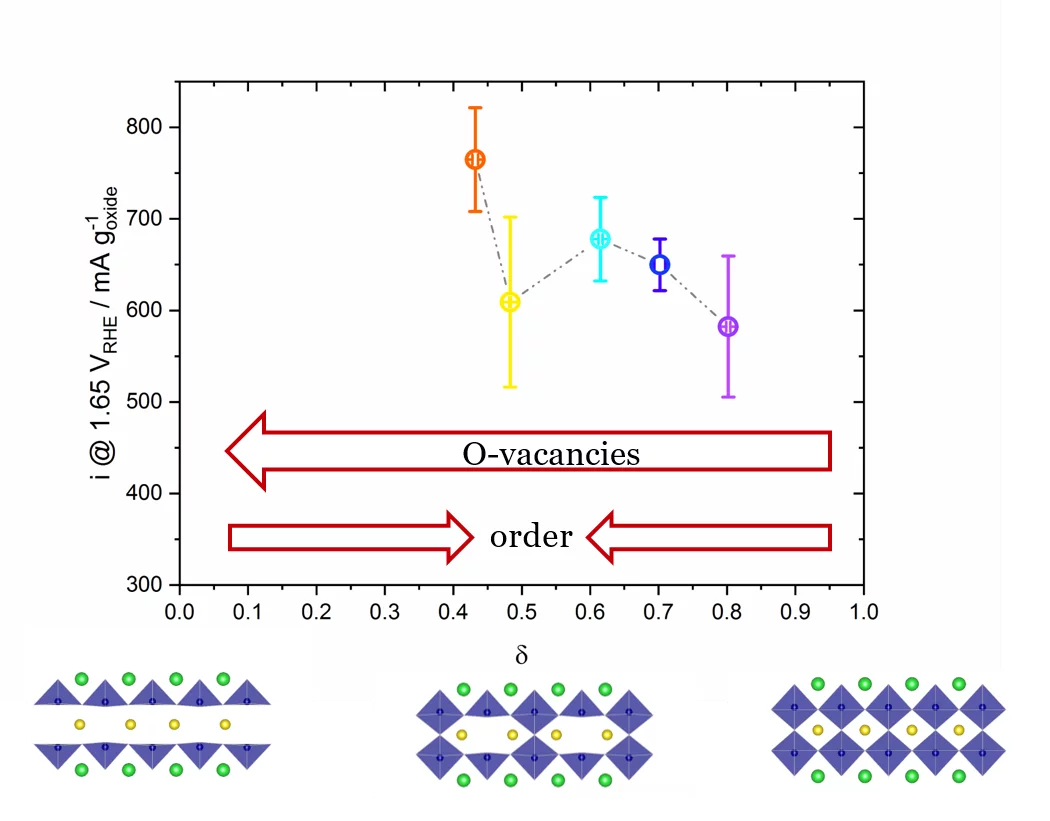
The role of the oxygen stoichiometry of perovskite catalysts in the oxygen evolution reaction (OER) is systematically studied in the PrBaCo2O5+δ family. The reduced number of physical/chemical variables combined with in-depth characterizations such as neutron diffraction, O K-edge X-ray absorption spectroscopy(XAS), electron energy loss spectroscopy (EELS), magnetization and scanning transmission electron microscopy (STEM) studies, helps investigating the complex correlation between OER activity and a single perovskite property, such as the oxygen content. Larger amount of oxygen vacancies appears to facilitate the OER, possibly contributing to the mechanism involving the oxidation of lattice oxygen, i.e., the lattice oxygen evolution reaction (LOER). Furthermore, not only the number of vacancies but also their local arrangement in the perovskite lattice influences the OER activity, with a clear drop for the more stable, ordered stoichiometry.
In recent years, great efforts have been dedicated to the understanding of the water-splitting mechanism and to the improvement of electrocatalyst materials. In particular, the anodic reaction, the oxygen evolution reaction (OER), is affected by slow kinetics and by significant overpotentials, even with the state-of-the-art electrocatalysts such as IrO2. The broad commercialization of green-hydrogen technologies urges for the use of highly active, stable and cost-effective electrocatalyst materials. Significant interest and promising results in alkaline environment came from perovskite-type oxides, where the large chemical flexibility allows for abroad variety of elemental compositions and fine properties tuning. Despite the great scientific interest and efforts, however, there is still no univocal consensus on the oxygen evolution reaction (OER) mechanism and on the perovskite electro-chemical contribution in the OER. The systematic study of the correlation between oxygen stoichiometry and OER activity in the PrBaCo2O5+δ materials shows an increase of OER activity as the lattice oxygen vacancies increase (see Figure 1). Furthermore, the trend of OER activity vs. oxygen vacancies content in not linear, indicating that other aspects should be considered to fully understand the key parameters of perovskite oxide catalysts affecting the OER. A clear activity drop is present for δ~0.5 (see Figure 1), where the oxygen-vacancies in the Pr plane orderforming alternating [CoO6] octahedral and [CoO5]square pyramids, with clear changes in the Co-O coordination and O-Co-O plane buckling. This suggests that oxygen vacancy ordering is detrimental for the OER activity of perovskites. The OER activity was correlated also with other physico-chemical properties of the perovskite materials, such as electronic conductivity, Co 3d–O 2p orbitals hybridization and electronic configuration, but with none of them, a clear correlation was observed.
Contact
Dr. Emiliana Fabbri, Scientist
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 27 95
E-mail: emiliana.fabbri@psi.ch
Original Publication
Temperature Dependent Water Transport Mechanism in Gas Diffusion Layers Revealed by subsecond Operando X-ray Tomographic Microscopy
Elena Marelli, Jaume Gazquez, Emiliya Poghosyan, Elisabeth Müller, Dariusz J. Gawryluk, Ekaterina Pomjakushina, Denis Sheptyakov, Cinthia Piamonteze, Dino Aegerter, Thomas J. Schmidt, Marisa Medarde, Emiliana Fabbri
Angew.Chem. Int. 60, 14609–14619 (2021)
DOI International Edition: 10.1002/anie.202103151
Acknowledgement
The authors gratefully acknowledge Paul Scherrer Institut CROSS initiative.