The PSI has over thirty years experience in the use of proton therapy. Our research laboratories developed the spot-scanning procedure that pinpoints and destroys tumours.
Innovative spot-scanning
The spot-scanning procedure developed by the Paul Scherrer Institute makes it possible to accurately irradiate tumours with a precisely defined radiation dosage. The spot-scanning technique is now used all over the world and has established itself internationally as the most promising development in proton therapy.
This method has been implemented at PSI since 1996. It is also called pencil- beam scanning, based on the fact that the proton beam is only as thick as a pencil: about five to seven millimetres.
Point by point, layer by layer
What makes spot-scanning special: the pencil-thin beam starts by scanning a given layer in the tumour. The beam is directed into all possible corners and recesses of that layer, developing its destructive impact spot by spot. The same process is carried out on the next layer and repeated, layer by layer, until the proton beam has spot-scanned through the entire tumour volume. About 10000 spots are required to scan a tumour with a volume of one litre.
Within this process, it’s possible to calibrate the radiation dosage very accurately: every point emits a precisely defined radiation dose. The pencil-thin beam scans through the tumour more than once. At PSI, tumours are irradiated from several directions (or fields).
As a result, spot-scanning has a number of advantages: proton beams adapt themselves with great precision to a tumour’s three-dimensional form. Healthy areas are barely affected. The distribution of spot doses within the tumour can also be adjusted depending on patient requirements.
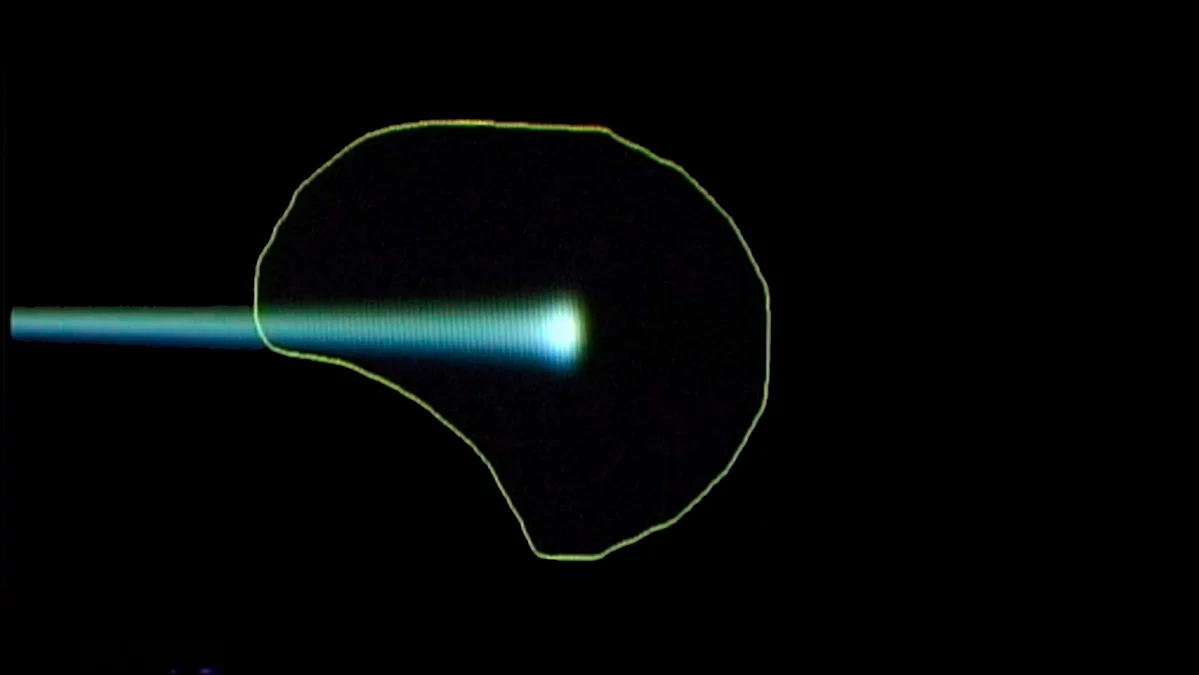
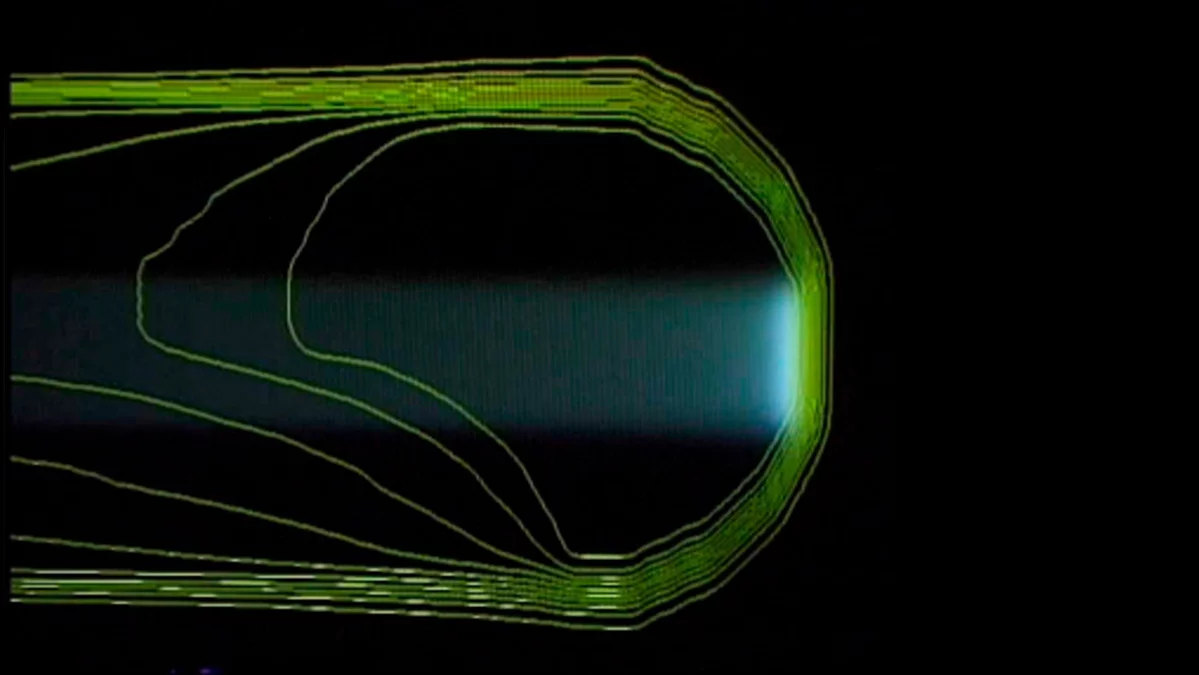
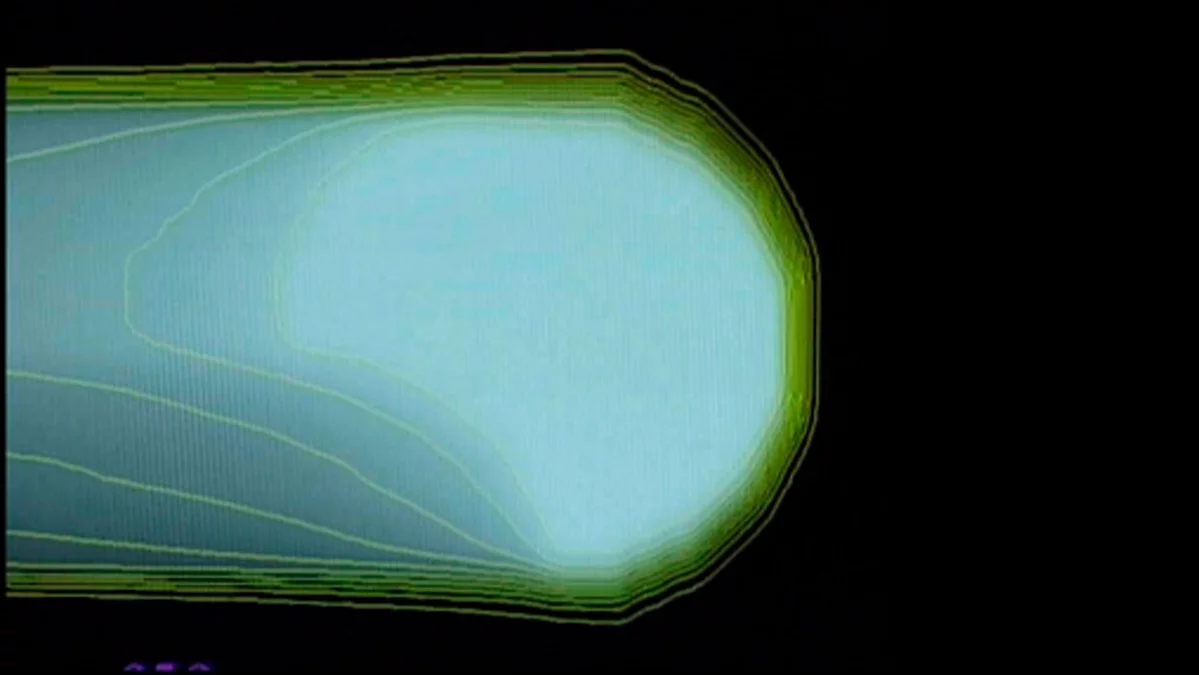
The principle of the spot-scanning technology developed at PSI
The three pictures show how the proton beam progressively encompasses the entire tumour volume. The first picture shows a single proton beam within the targeted area outlined in yellow, schematically representing the tumour. The beam emits its maximum dose at the end of its trajectory. Bit by bit, layer by layer, the beam scans through the targeted area. As can be seen on the third picture, the entire tumour is then targeted with the maximum dosage. To the left of the tumour, the proton beam gives a lower dose than within the target; there is no more proton radiation on the right-hand side of the picture, beyond the edge of the tumour.
A further advantage of intensity-modulated proton therapy is the ability to perform a dose boost to the tumour. This is the term given by radiation oncologists to the extra dose of radiation directed at a small area within the tumour in which the danger of recurrence is greatest. Generally, the boost is administered at the end of treatment.
PSI as pioneer
Proton therapy has a long tradition at the Paul Scherrer Institute: doctors and medical physicists working in Villigen in the Swiss Canton of Aargau began irradiating ocular tumours with highly successful courses of proton treatment as early as 1984.
From 1996 onwards, we expanded our therapy to include treatment of deep-seated tumours. Since that time, patients have benefited from the spot-scanning procedure invented by PSI, which destroys tumours with a finely modulated beam. Since 2004, we have been able to treat infants (under anaesthetic) with this therapy. A team of anaesthetists from the Zurich Children’s Hospital (Kinderspital Zürich) is responsible for administering anaesthetics to children undergoing treatment at PSI.
Combined strength: clinic and research
The Center for Proton Therapy (CPT) is part of the PSI research facility. As such, we guarantee state-of-the-art, on-site expertise based on our continued research into and development of new technical procedures aimed at improving cancer treatments. At the same time, your needs as a cancer patient, as well as the results of medical evaluations, continue to inform our research at CPT. Patients at CPT receive qualitatively outstanding treatment. The therapy is reliable and effective, and is carried out under strict supervision.
Proton therapy research for patients
The Paul Scherrer Institute leads the field in research. Scientists are dedicated above all to progress in the field of proton therapy with particular emphasis on the question: how is optimum treatment possible without damaging healthy tissue?
At PSI scientists are exploring the best approach to treating moving tumours with protons. Moving tumours are those that change position minimally as a result of breathing and can include lung or breast cancer tumours.
We are continually evaluating clinical patient data within the framework of scientific projects. We also carry out studies on quality of life after proton therapy or on other specific, therapy-linked issues. We welcome the participation of any patient willing to take part in these studies, which are carried out according to Switzerland’s strict legal guidelines. Such studies serve as an ongoing quality control as well as improving the range of therapies we are able to offer. Study results are published in scientific journals and presented at specialist conferences.
Successful treatments: the numbers
By the end of 2022 PSI had treated over 8000 patients with ocular tumours and more than 2000 with deep-seated tumours. Among them more than 770 children and young persons were able to benefit from this more sparing form of therapy.
In over 98 per cent of ocular cancers treated in Villigen since 1984, tumour growth was arrested. In over 90 per cent of these cases, the affected eye was saved.
In contrast to ocular cancers, the deep seated tumours constitute a more diverse group. Successful treatment depends largely on the location, size and type of tumour, as well as on various forms of pre-treatment. For example: a given course of cancer treatment results in long-term tumour control in up to 90 per cent of cases, whilst for other types of tumour 66 per cent of patients can expect at least five years of tumour control.
Great technology for small particles
The centrepiece of the PSI’s proton therapy facility is the particle accelerator COMET (COmpact MEdical Therapy Cyclotron). The accelerator weighs 90 metric tons and supplies all treatment areas with protons.
COMET accelerates protons to a speed of about 60 per cent of the speed of light. This corresponds to about 180,000 km per second. During this procedure, the particles circle around the cyclotron ring several hundred times, picking up speed and energy. They are subsequently catapulted out of the cyclotron, bundled and slowed down where necessary. At PSI, this part of the process is carried out by so-called “degraders” or brake plates, which are inserted into the radiation beam. Magnetic fields direct the beam to the respective radiation treatment rooms.
A sophisticated, five-step control system carries out checks every hundredth of a microsecond to ensure that proton beams are being correctly directed. In this way, the safety of the cyclotron, the distribution system and the treatment areas are guaranteed.