Recent highlights
Research activities
Our first goal is to develop novel materials and to improve existing materials for Li/Na-ion batteries. We apply different synthetic routes, such as sol-gel chemistry, mechano-synthesis, solid-state synthesis under various controlled atmospheres, and microwave synthesis to control the morphology and to obtain the most promising electrochemical performances of the investigated systems. We have also the capability of realizing thin film growth of solid electrolyte, cathode and anode materials for applications e.g. in micro-batteries or to be used as model electrodes. The thin films growth are performed in our UHV cluster connected to the XPS spectrometer and equipped with RF sputtering, e-gun evaporators under various gas atmosphere.
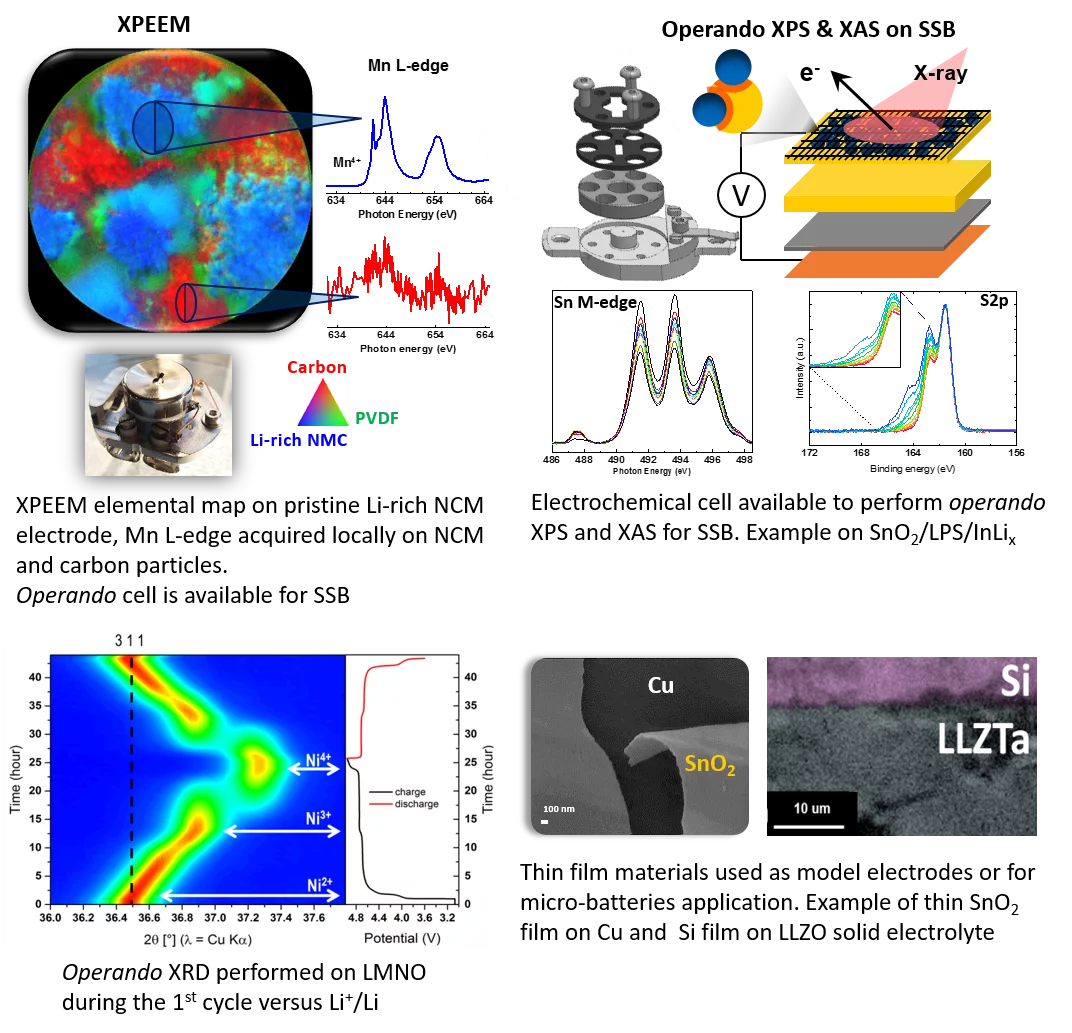
Our second goal is to elucidate the electrochemical reaction mechanisms by applying post mortem or operando measurements by combining a wide range of techniques in-house or at big facilities dedicated to surface and bulk characterization. We have developed electrochemical cells for liquid-based and all-solid-state batteries for operando X-ray diffraction, neutron diffraction, X-ray absorption spectroscopy (XAS in transmission, TEY and TFY modes), X-ray tomography, X-ray photoemission electron microscopy (XPEEM), X-ray photoemission spectroscopy (XPS) etc. Many of our activities are supported by our industrial partners.
Open positions
The BMD group offers the opportunity to conduct thesis and semester projects in the field of Li-ion all-solid-state batteries and their bulk and surface in-situ/operando characterization. Currently available research topics focus on surface modifications of the electrode-electrolyte interface to enhance battery performance, "thin Li metal" or "Li-reservoir free" anodes applications and the study of chemo-mechanical phenomena at the battery interfaces.
In particular we offer a master thesis project on operando X-ray photoelectron spectroscopy to study the electrode-electrolyte interface during battery operation. In the frame of such project you will learn how to build all-solid-state batteries and evaluate their electrochemical performance in regard of their degradation phenomena. You will learn how to characterize solid-solid interfaces by XPS and complement your findings by SEM, EDX, ion-milling and XRD. Moreover, you will learn how to work in an electrochemistry laboratory and how to operate on an Argon filled glovebox.
We encourage bachelor and master students who are interested in conducting their thesis or semester project in our lab to contact our postdoctoral researcher Valerie Siller via email: valerie.siller@psi.ch
Please provide a C.V. and a short description of your motivation for this application, desired period of time and briefly state your current experience of working in a laboratory or with any of the techniques mentioned above (if applicable).
Group members
- Dr. Mario El Kazzi, Group Head, Google Scholar, ORCID
- Dr. Dominika Baster, Scientist
- Barthélémy Lelotte, PhD student (joint project with SAFT)
- Linfeng Xu, PhD student (SNF-Sinergia)
- Jinsong Zhang, PhD student (SNF-Sinergia)
- Dr. Valerie Siller, Postdoc (joint project with SIM beamline at SLS) (SFA-AM)
- Dr. Ramasamy Hari Vignesh, Postdoc (Innosuisse project)
- Adil Baiju, PhD student (joint project between ENE, NUM & PSD)
- Robin Wullich, PhD student
Former group members
PhD students and Postdocs
- Dr. Tian Liu, 2021-2023 - IVECO Group Switzerland
- Dr. Santhosha Aggunda Lingamurthy, 2022 - SAFT, France
- Dr. Ales Stefancic, 2019-2021 - Belenos, Switzerland
- Dr. Steven Lacey, 2019-2021 - Postdoc at Huntsman Advanced Materials - Switzerland
- Dr. Juliana Bruneli Falqueto, 2020-2021 - Postdoc at Paul Scherrer institute, Switzerland
- Dr. Laura Höltschi, 2016-2021 - Leclanché
- Dr. Marta Mirolo, 2016-2020 - Postdoc at ESRF - ID31, Grenoble France
- Dr. Xiaohan Wu, 2016-2019 - Porsche consulting, Germany
- Dr. Daniela Leanza, 2015-2018 - R&D Battery Engineer at Sonova group, Switzerland
- Dr. Giulio Ferraresi, 2014-2018 - Hilti, Liechtenstein
Master/Semester Students and Visitors
- Robin Wullich, 2022 - ETH-Zurich
- Ernesto Claure Ramirez, 2022 - Friedrich-Alexander Universitaet Erlangen-Nuernberg
- Adil Baiju, 2022 - ETH-Zurich
- Bowen Li, 2022 - Technology University of Denmark-DTU
- Joel Handschin, 2021 - ETH-Zurich
- Moritz Bohn, 2020 - Technical University Munich (TUM)
- Gabriel Quintans, 2020 - IAESTE, Royal Institute of Technology, Sweden
- Keisuke Morita, 2019 - Toyota Motor Corporation, Japan
- Nick Seiller, 2018
- Dr. Alice Judith Gillen, 2014 - Lawrence Livermore National Laboratory
Recent publications
-
Falqueto JB, Clark AH, Kondracki Ł, Bocchi N, El Kazzi M
Unveiling the (de-)lithiation mechanism of nano-sized LiMn2O4 allows the design of a cycling protocol for achieving long-term cycling stability
Journal of Materials Chemistry A. 2023; 11: 24800-24811. https://doi.org/10.1039/D3TA04660E
DORA PSI -
Lacey SD, Gilardi E, Müller E, Merckling C, Saint-Girons G, Botella C, et al.
Integration of Li4Ti5O12 crystalline films on silicon toward high-rate performance lithionic devices
ACS Applied Materials and Interfaces. 2023; 15(1): 1535-1544. https://doi.org/10.1021/acsami.2c17073
DORA PSI -
Falqueto JB, Clark AH, Štefančič A, Smales GJ, Vaz CAF, Schuler AJ, et al.
High performance doped Li-rich Li1+xMn2-xO4 cathodes nanoparticles synthesized by facile, fast, and efficient microwave assisted hydrothermal route
ACS Applied Energy Materials. 2022; 5(7): 8357-8370. https://doi.org/10.1021/acsaem.2c00902
DORA PSI -
Wu X, Mirolo M, Vaz CAF, Novák P, El Kazzi M
Reactivity and potential profile across the electrochemical LiCoO2-Li3PS4 interface probed by operando X-ray photoelectron spectroscopy
ACS Applied Materials and Interfaces. 2021; 13(36): 42670-42681. https://doi.org/10.1021/acsami.1c09605
DORA PSI -
Höltschi L, Borca CN, Huthwelker T, Marone F, Schlepütz CM, Pelé V, et al.
Performance-limiting factors of graphite in sulfide-based all-solid-state lithium-ion batteries
Electrochimica Acta. 2021; 389: 138735 (10 pp.). https://doi.org/10.1016/j.electacta.2021.138735
DORA PSI -
Mirolo M, Wu X, Vaz CAF, Novák P, El Kazzi M
Unveiling the complex redox reactions of SnO2in Li-Ion batteries using operando X-ray photoelectron spectroscopy and in situ X-ray absorption spectroscopy
ACS Applied Materials and Interfaces. 2021; 13(2): 2547-2557. https://doi.org/10.1021/acsami.0c17936
DORA PSI -
Leanza D, Vaz CAF, Novák P, El Kazzi M
Instability of PVDF binder in the LiFePO4 versus Li4Ti5O12 Li‐Ion battery cell
Helvetica Chimica Acta. 2021; 104(1): e2000183 (9 pp.). https://doi.org/10.1002/hlca.202000183
DORA PSI -
Höltschi L, Jud F, Borca C, Huthwelker T, Villevieille C, Pelé V, et al.
Study of graphite cycling in sulfide solid electrolytes
Journal of the Electrochemical Society. 2020; 167(11): 110558 (10 pp.). https://doi.org/10.1149/1945-7111/aba36f
DORA PSI -
Vaz CAF, Kleibert A, EL Kazzi M
Nanoscale XPEEM spectromicroscopy
In: Sattler KD, ed. Advanced analytic methods and instrumentation. 21st century nanoscience – a handbook. Boca Raton: Taylor & Francis; 2020:17 (21 pp.). https://doi.org/10.1201/9780429340420-17
DORA PSI -
Mirolo M, Vaz CAF, Novák P, El Kazzi M
Multi-length-scale x-ray spectroscopies for determination of surface reactivity at high voltages of LiNi0.8Co0.15Al0.05O2 vs Li4Ti5O12
Journal of Chemical Physics. 2020; 152(18): 184705 (13 pp.). https://doi.org/10.1063/5.0006269
DORA PSI -
Wu X, Villevieille C, Novák P, El Kazzi M
Insights into the chemical and electronic interface evolution of Li4Ti5O12 cycled in Li2S-P2S5 enabled by operando X-ray photoelectron spectroscopy
Journal of Materials Chemistry A. 2020; 8(10): 5138-5146. https://doi.org/10.1039/C9TA14147B
DORA PSI -
Mirolo M, Leanza D, Höltschi L, Jordy C, Pelé V, Novák P, et al.
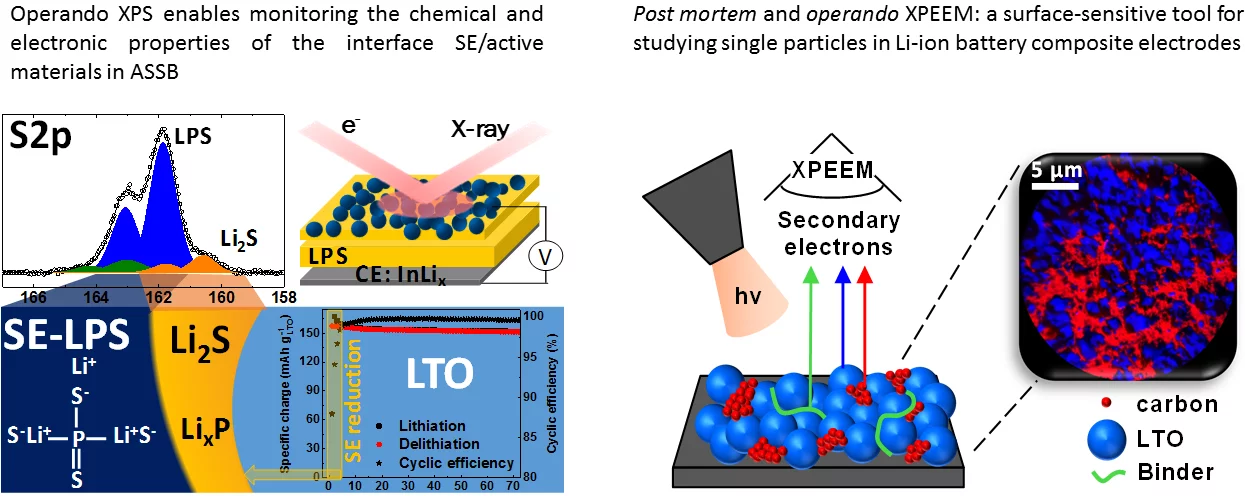
Post mortem and operando XPEEM: a surface-sensitive tool for studying single particles in Li-Ion battery composite electrodes
Analytical Chemistry. 2020; 92(4): 3023-3031. https://doi.org/10.1021/acs.analchem.9b04124
DORA PSI -
Leanza D, Mirolo M, F. Vaz CA, Novák P, El Kazzi M
Surface degradation and chemical electrolyte oxidation induced by the oxygen released from layered oxide cathodes in Li−ion batteries
Batteries and Supercaps. 2019; 2: 482-492. https://doi.org/10.1002/batt.201800126
DORA PSI -
Leanza D, Vaz CAF, Melinte G, Mu X, Novák P, El Kazzi M
Revealing the dual surface reactions on a HE-NCM Li-Ion battery cathode and their impact on the surface chemistry of the counter electrode
ACS Applied Materials and Interfaces. 2019; 11(6): 6054-6065. https://doi.org/10.1021/acsami.8b19511
DORA PSI -
Ferraresi G, El Kazzi M, Czornomaz L, Tsai C-L, Uhlenbruck S, Villevieille C
Electrochemical performance of all-solid-state Li-ion batteries based on garnet electrolyte using silicon as a model electrode
ACS Energy Letters. 2018; 3(4): 1006-1012. https://doi.org/10.1021/acsenergylett.8b00264
DORA PSI -
Ferraresi G, Villevieille C, Czekaj I, Horisberger M, Novák P, El Kazzi M
SnO2 model electrode cycled in Li-Ion battery reveals the formation of Li2SnO3 and Li8SnO6 phases through conversion reactions
ACS Applied Materials and Interfaces. 2018; 10(10): 8712-8720. https://doi.org/10.1021/acsami.7b19481
DORA PSI -
Leanza D, Vaz CAF, Czekaj I, Novák P, El Kazzi M
Solving the puzzle of Li4Ti5O12 surface reactivity in aprotic electrolytes in Li-ion batteries by nanoscale XPEEM spectromicroscopy
Journal of Materials Chemistry A. 2018; 6(8): 3534-3542. https://doi.org/10.1039/c7ta09673a
DORA PSI -
Sun B, El Kazzi M, Müller E, Berg EJ
Toward high-performance Li(NixCoyMnz)O2 cathodes: facile fabrication of an artificial polymeric interphase using functional polyacrylates
Journal of Materials Chemistry A. 2018; 6(36): 17778-17786. https://doi.org/10.1039/C8TA03954B
DORA PSI -
Wu X, Villevieille C, Novák P, El Kazzi M
Monitoring the chemical and electronic properties of electrolyte-electrode interfaces in all-solid-state batteries using operando X-ray photoelectron spectroscopy
Physical Chemistry Chemical Physics. 2018; 20(16): 11123-11129. https://doi.org/10.1039/c8cp01213j
DORA PSI -
Ferraresi G, Czornomaz L, Villevieille C, Novák P, El Kazzi M
Elucidating the surface reactions of an amorphous Si thin film as a model electrode for Li-ion batteries
ACS Applied Materials and Interfaces. 2016; 8(43): 29791-29798. https://doi.org/10.1021/acsami.6b10929
DORA PSI -
El Kazzi M, Czekaj I, J. Berg E, Novák P, Brown MA
Investigation of Li-ion solvation in carbonate based electrolytes using near ambient pressure photoemission
Topics in Catalysis. 2016; 59(5-7): 628-634. https://doi.org/10.1007/s11244-015-0518-2
DORA PSI