The fixed-targets that SwissMX typically support are: the PSI MISP (MIcro-Structured Polymer) chip (Carrillo et al., 2023) and the Max Plank Institut (MPI) SOS (Sheet-On-Sheet) chip (Doak et al., 2018), although other targets such as the Oxford (Horrell et al., 2021) and HARE chips (Mehrabi et al., 2020) could also be used. The TELL sample changer can be used to remotely store (at 80 % relative humidity) and exchange the MISP chip during a beamtime to increase beamtime efficiency.
Fixed-target data-collection
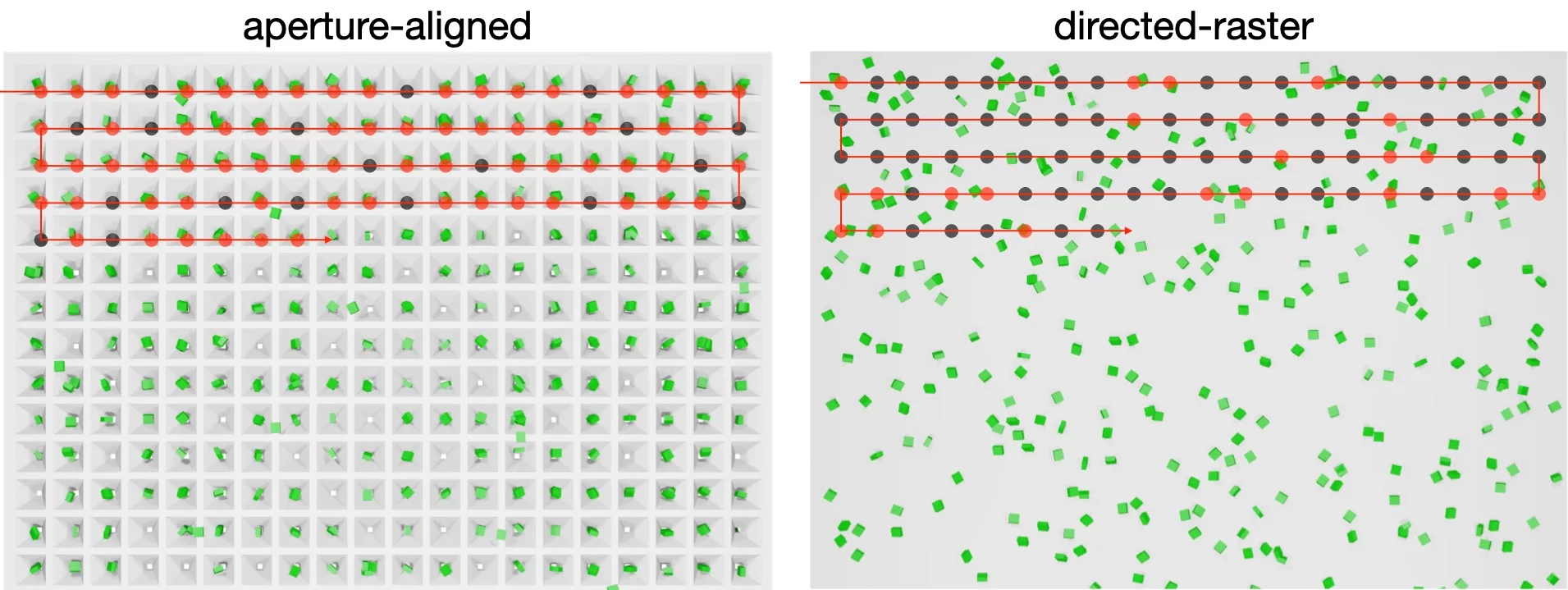
Fixed-targets can enable two types of data-collection: aperture-aligned, where crystals are loaded into precisely positioned cavities at known locations and sequentially exposed, or, directed-raster, where a raster grid is defined over crystals in completely random locations.
The PSI MISP chips
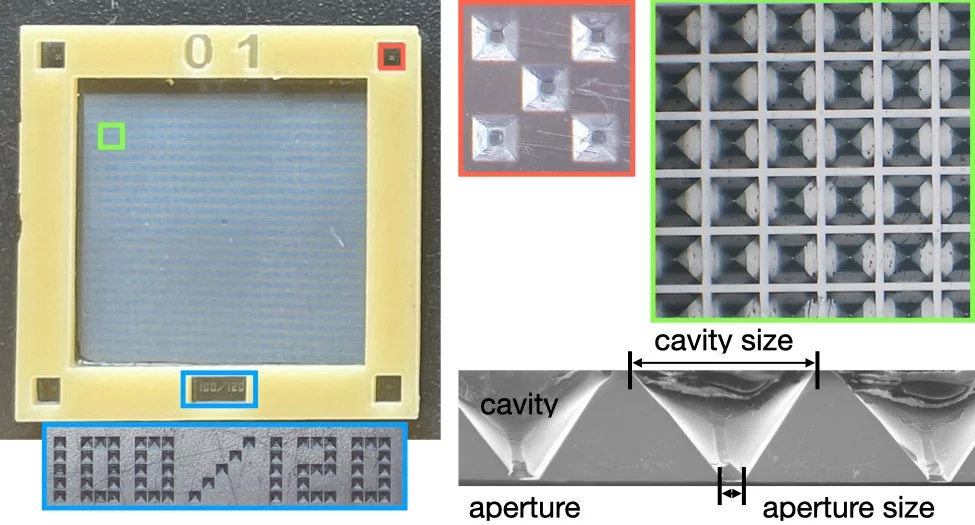
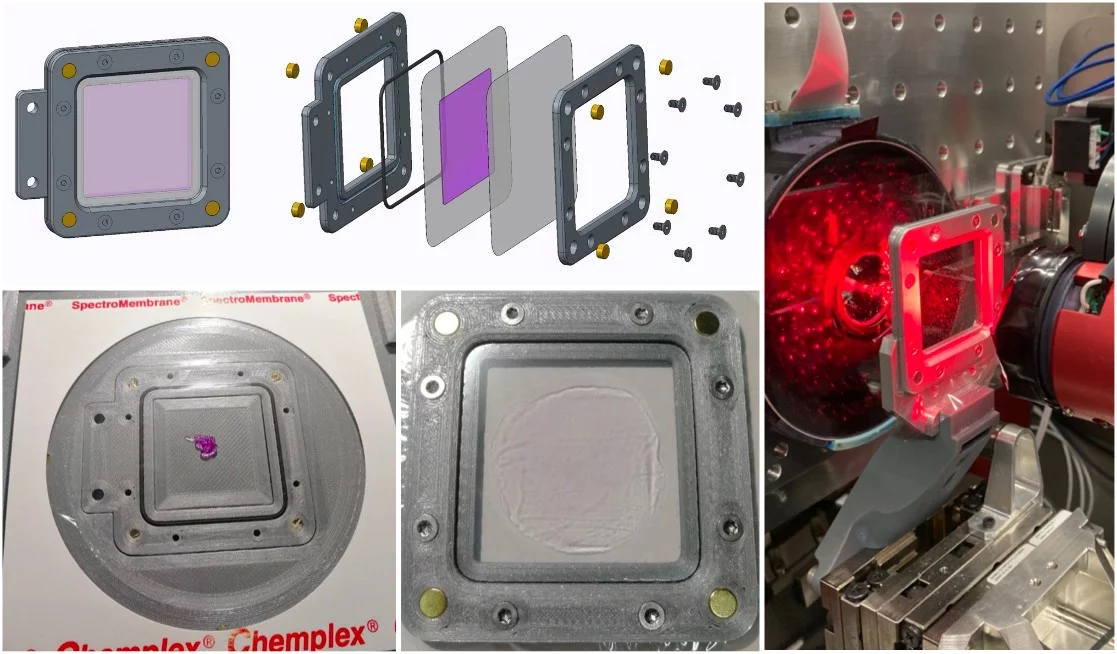
The MIcro-Structure Polymer (MISP) fixed-targets were developed for the SwissMX endstation through a collaboration between the Laboratory for Macromolecules and Bioimaging (LSB) and the Laboratory for X-ray Nanoscience and Technologies (LXN) in the Photon Science Division (PSD) and the Laboratory of Nanoscale Biology (LNB) in the Division of Biology and Chemistry (BIO) at PSI. The chips are an attempt to combine the precision of silicon micro-fabrication technologies with a low-cost polymer materials. Like the Oxford and HARE chips, the MISP-chips are composed of a precise array of apertures. Protein crystals are loaded into these apertures by pipetting a micro-crystal solution to the surface and applying a vacuum or blotting away the excess solution from the underside. Once the the majority of the solution is removed, the chip is sealed between two pieces of film in a holder. Once loaded on the beamline, the apertures are rapidly rastered through in step with the XFEL pulse (this video from Diamond Light Source gives a nice overview of the process).
The MISP-chips are currently fabricated in two sizes and from two materials (transparent COC and opaque COP). The sizes are defined by the fiducial spacing and are currently: 12.5 and 23.0 mm. The opaque COP chips are necessary for laser pump-probe experiments to ensure that the pump light is not transmitted through the chips and contaminates adjacent cavities. The opaque COP chips, due to the opaque film fabrication, have slightly different cavity properties. The table below gives a summary of these.
| transparent | opaque | ||
| material | COC | COP | |
| measurement type | SFX | SFX pump-probe | |
| fiducial width (µm) | 12.5 | 23.0 | 23.0 |
| pitch (µm) | 120.0 | ||
| cavity size (µm) | 100.0 | ||
| mean aperture size (µm) | 6.3 ± 1.0* | 8 ± 3.0† | |
| minimum crystal size (µm) | > 5.0 | > 10.0 | |
(*) Lysozyme crystals with a longest dimension as small as 4 µm have been caught in the chip apertures.
(†) It is very challenging to load single crystals into single wells as the crystal size decreases. An ideal crystal size for pump-probe in these chips is currently approximately 15-30 µm. Outside of these limits, the likelihood of crystal pump excitation decreases as either the number of crystals in the cavity increases or the crystal size limits the excited fraction. This is not to say it is impossible, but certainly not ideal.
MPI SOS fixed-targets
The SOS support was developed by the MPI as a cheap and highly effective way of mounting samples for serial crystallography measurements utilising a directed-raster style of data collection (Doak et al., 2018). These supports are a much more refined version of the thin-film sandwiches initially developed for use at the synchrotron (Huang et al., 2015, Axford et al., 2016). The key advance, other than the holder design, was the loading technique to ensure a minimal and uniform sample thickness across the thin-film sandwich over a large area.
Recent commissioning has shown that a 25 x 25 µm grid (collected at 12 keV) yields undamaged data. The SOS supports with this spacing allow for a significant quality of data to be collected from a small area (250,000 images from 12.5 x 12.5 mm2); maximising the amount of data collected from the minimal amount of sample (8 µL). Smaller spacing may be possible but have yet to be tested.
The ideal samples for the SOS chips are either crystals grown in the Lipidic Cubic Phase (LCP) or concentrated solutions of micro-crystals that have been mixed with 2-5 % (w/v) ethyl cellulose making them moderately viscous. Crystals loaded simply in solution are liable to migrate as the chip is mounted due to gravity. Otherwise, the SOS supports are incredibly versatile, happily accepting both very small (≥1 µm) and large (>50 µm) crystal samples. Given this, and the small volumes required, the SOS chips are an ideal support for SFX measurements. SFX pump-probe using the SOS fixed-targets is currently the subject of commissioning but currently not advised.
Pump-probe measurements
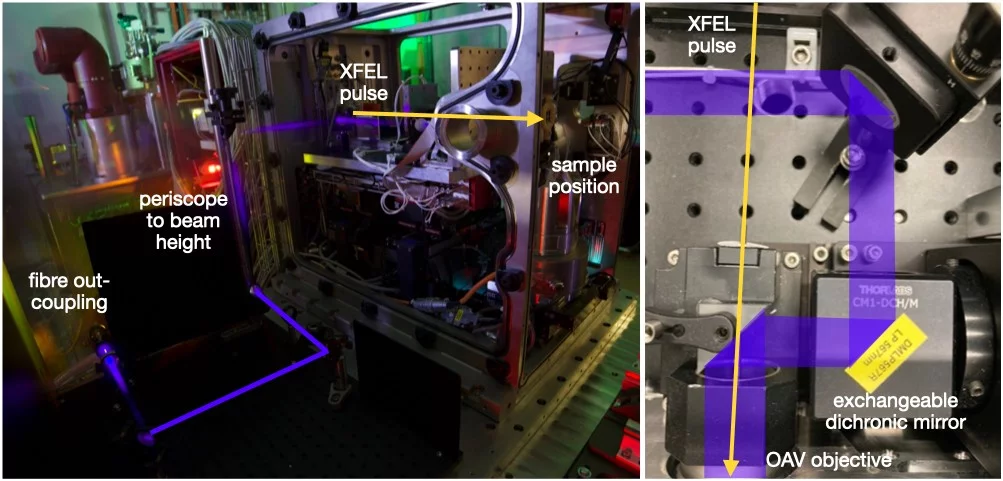
Laser coupling
The SwissMX can be fibre coupled to EKSPLA nanosecond OPO located in the laser hutch (see Pump-laser at Cristallina-MX). The fibre coupling limits the usable laser energy achievable from the fibre to 5-10 µJ as Raman shifts have been observed at higher pulse energies; although, this is wavelength dependent. From the fibre, the laser is coupled to the sample position via the objective of the SwissMX on-axis viewing (OAV) system; co-aligning the X-ray and pump laser pulses.
Fixed-targets available for pump-probe
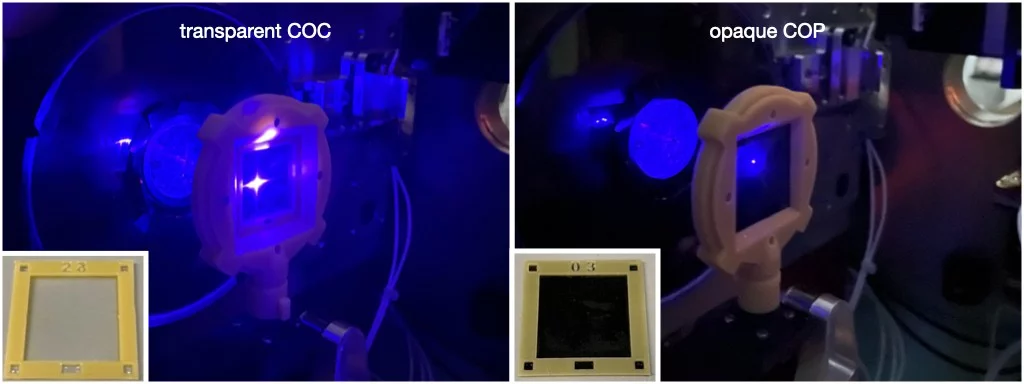
The opaque MISP-chips are the only fixed-targets we provide that are currently suitable for SFX pump-probe measurements using the SwissMX. The image shows a comparison between the transparent and opaque chips. As can be seen, the light is highly transmitted through the transparent chips. Initial commissioning SFX pump-probe experiments, now published in Gotthard (2024), confirmed that the cavity-to-cavity light contamination using the transparent chips was extensive whereas the opaque chips remove this problem. In this study, zero light contamination was only observe in schemes where the open cavity was directed away from the laser. Subsequent improvements of the instrumentation have now enabled contamination-free data-collection in the opposite chip orientation. This observation has been confirmed in commissioning, pilot and now user experiments (references hopefully to follow soon).
Available time delays
Two stage-motion routines are now available (July 2024): continuous and stop-go and these can be used effect different pump-probe delays. In continuous motion, as the name suggests, the stages do not stop at each aperture, but time their arrival precisely with the XFEL pulse. This means that data-collection rates can be maintained at 100 Hz, but are only applicable for time-delays < 1 ms. If you need longer delays, then you need to use stop-go where the stages move to an aperture and stop for a given number of pulses. This routine enables delays of > 1 ms, however, it will become very beamtime inefficient beyond 40-50 ms as the effective data rate drops proportionally with the delay, e.g., a 40 ms delay (+ 10 ms move) drops the data-collection rate to 20 Hz.
The implementation of hit-and-return type routines (Schulz et al., 2018) is a priority for the end of 2024 and we hope to have these commissioned for 2025.
| pulse-width (FWHM) | 3.2 ns (FWHM) at 450 nm |
| maximum pulse energy | 2 µJ |
| commissioned wavelengths | 410 - 700 nm |
| laser profile | 30 x 30 µm2 at 450 nm |
| ΔT range | 50 ns - 40 ms |
| fixed-targets | opaque MISP-chips |
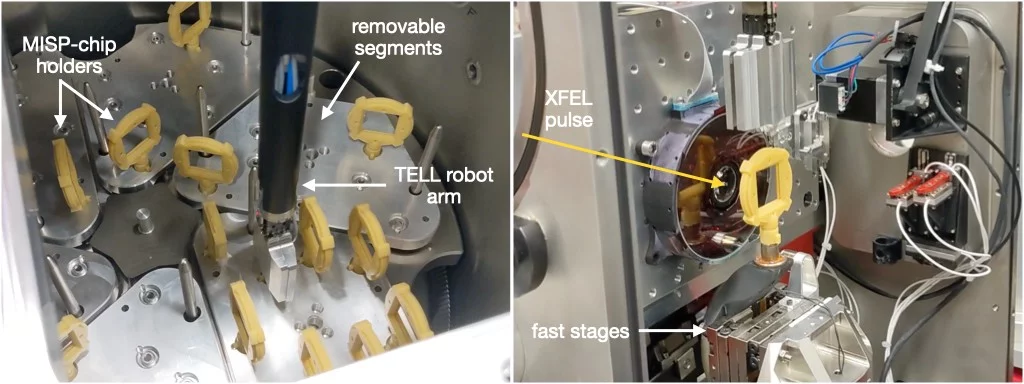
TELL sample changer
To increase the throughput of the SwissMX, a TELL robot system has been adapted to work with the large area fixed-targets and a humidified storage container. The relative humidity of the chamber is set to 80 %, a level found to be sufficient for maintaining the diffraction potential and number of crystals in a fixed-target for a series of test proteins [lysozyme, thaumatin, bacteriorhodopsin in LCP and insulin (Huang et al., 2022)] for up to seven days. The survival of a variety of protein crystals during commissioning using the humidified storage system appears to have born out these initial findings. The system simplifies and speeds up the process of sample exchange as the continual opening and searching of the hutch is dramatically minimised when the TELL can be employed. Sample preparation is also improved as 10-20 MISP chips can be prepared at once and safely stored until data-collection.
The use of the TELL robot is currently only possible using the MISP chips. The SOS chips use a different fixation method to the fast-stages making them incompatible with the robot. However, since SOS chip data-collections are typically long (250,000 images at 100 Hz = 41.7 min) compared to a 23.0 mm MISP chip (26,000 images at 100 Hz = 4.3 min ), the need for rapid sample exchange of the SOS chip is reduced.