Precipitation is mostly formed via the ice phase in mixed phase clouds and ice clouds are very relevant for Earths’ climate. Ice nucleation is the first step in freezing. Freezing or prevention of freezing is common to everyday life, e.g. for food and drug storage, icing and de-icing, etc. However, the ice nucleation process is not well understood, since it occurs on the size scale of clusters of molecules and time scales of molecular fluctuations.
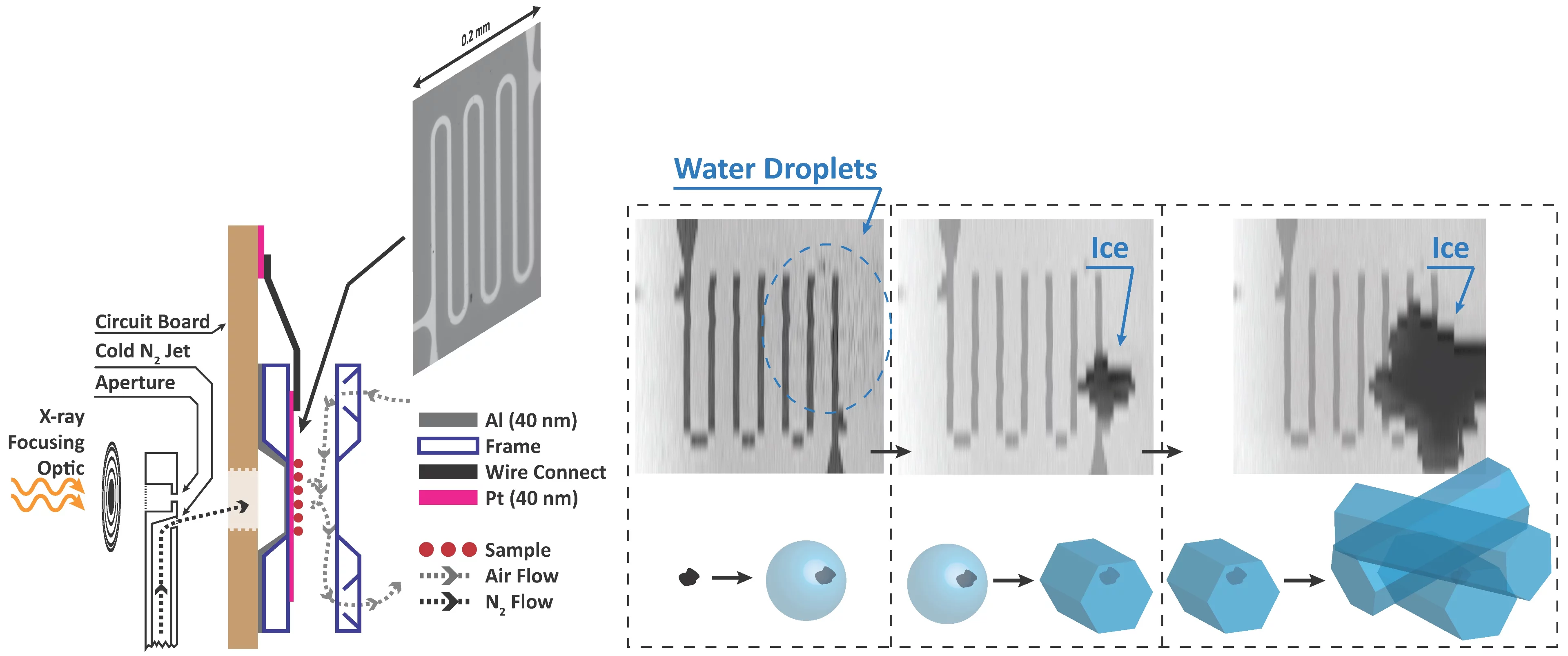
We built new instrumentation to observe when ice forms and use X-ray microscopy and spectroscopy to track the physical and chemical properties of ice nucleating particles with 35 nm spatial resolution. This is the first time ice nucleation has been measured in-situ in an X-ray microscope. The main technical challenge was temperature, and thus relative humidity control, while maintaining X-ray transparency. A sketch of our setup is shown. In a standard configuration, X-rays are focused through an aperture onto our sample. We have modified the aperture to also host a jet of nitrogen and control its temperature down to 170 K. The cold jet is directed to the back of an environmental cell mounted on a circuit board. Inside the cell with front and back windows (~100 nm thin), our sample is exposed to water vapour. The temperature of the sample is measured using a custom microfabricated sensor patterned using electron-beam lithography. Finally, the cold jet cools the sample only on the window exactly where we want ice nucleation to occur. An example of ice nucleation on iron containing nano-particles, ferrihydrite, in shown in the figure. This iron mineral is contained in mineral dust, which itself is assumed to be ice active. We can distinguish first, when water drops appear (blurred area), when ice first appears and grows, and measure the corresponding temperature and humidity.
This spot-cooling technique can be used for a wide range of applications and include, in part, determining ice nucleating properties of natural aerosol samples or observing chemical changes due to reaction in aerosol particles at low temperature. As a user instrument at the PolLux endstation of the SLS, this will be available to a variety of scientists in many disciplines.
Contact
Dr. Peter A. Alpert
PostDoc at the Surface Chemistry Research Group
Paul Scherrer Institute PSI, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 3934; E-mail: peter.alpert@psi.ch