Surface Analytical Techniques
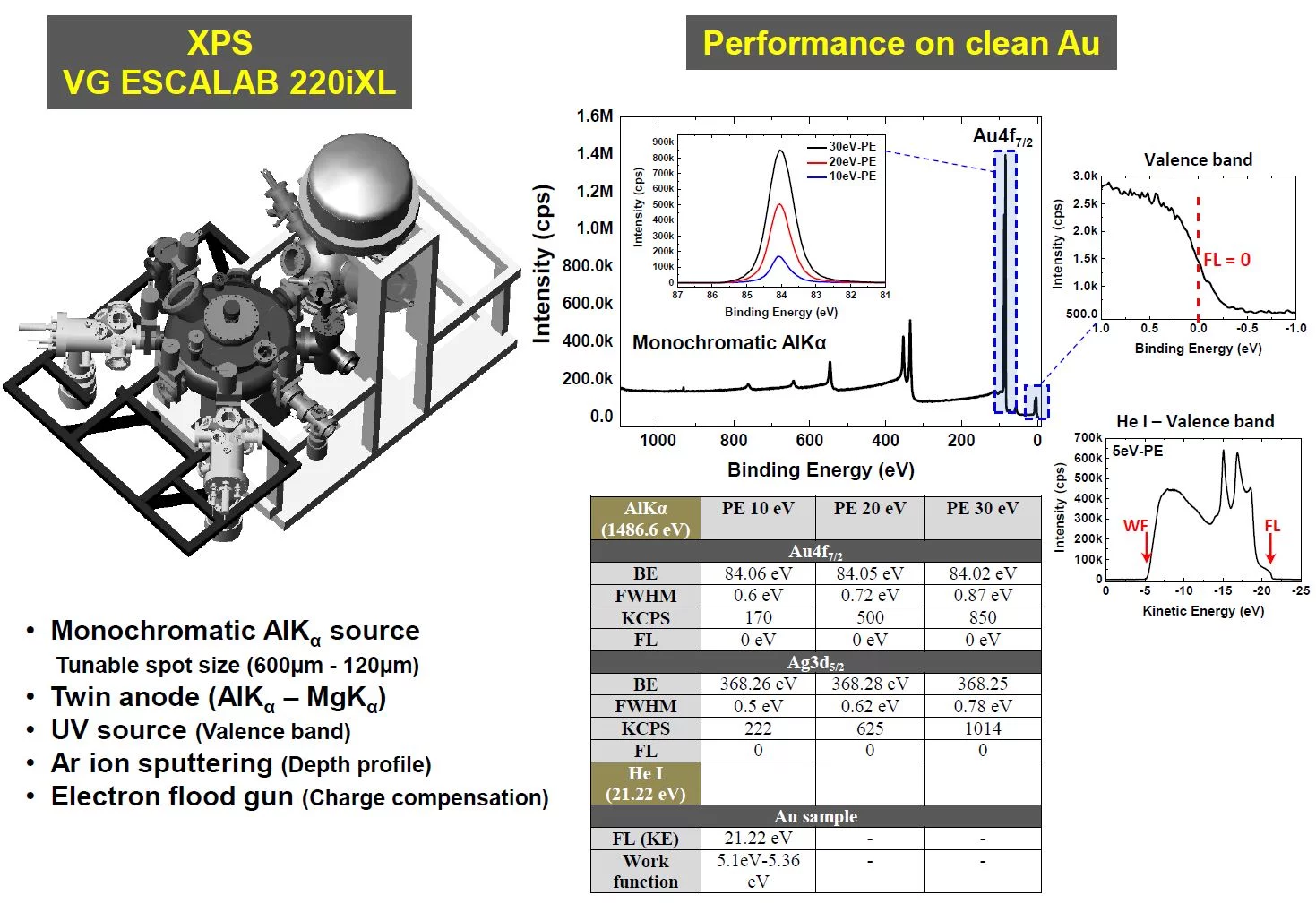
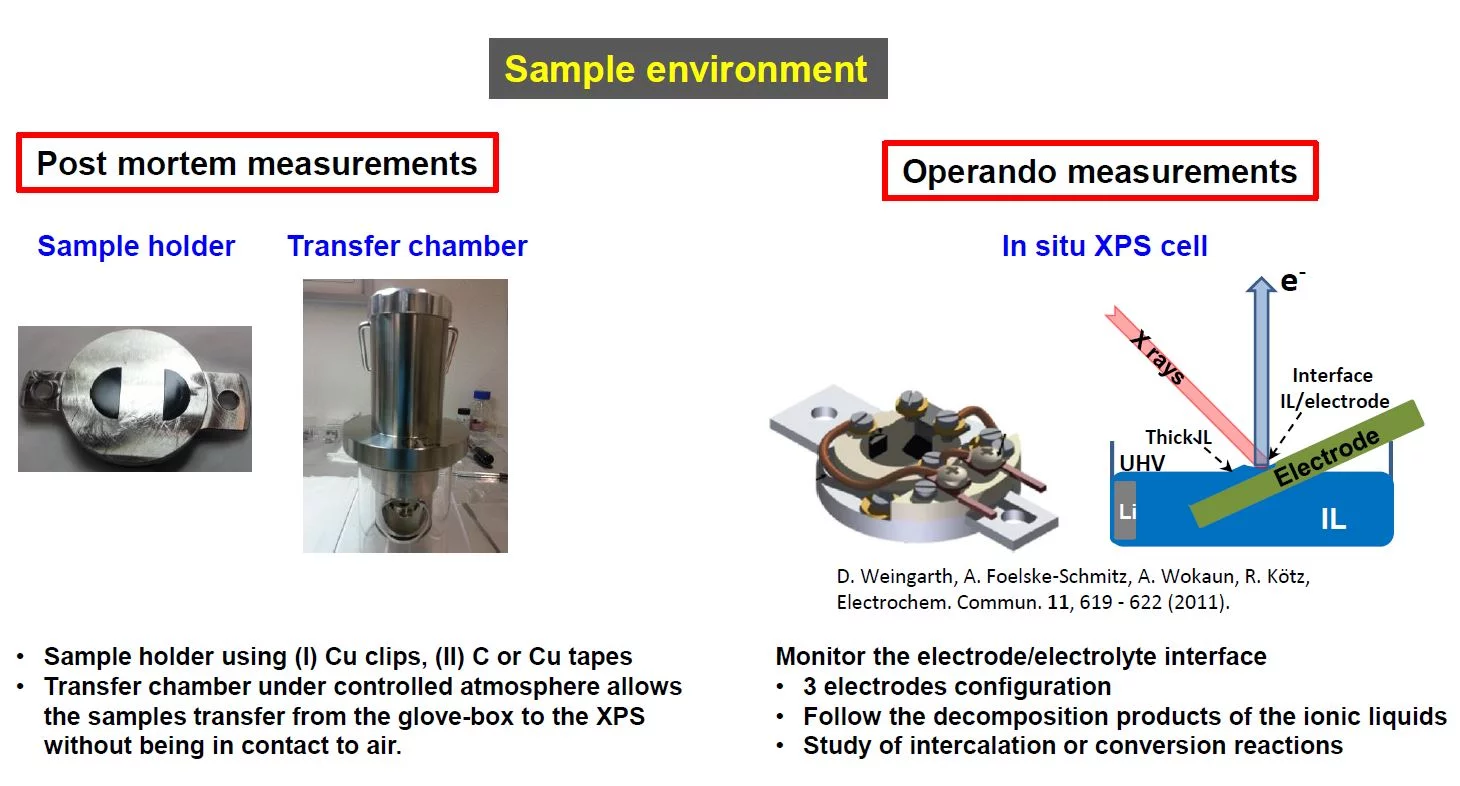
X-ray photoemission spectroscopy (XPS)
For more information: Advanced Characterization
in situ Bulk Analytical Techniques
X-ray powder diffraction
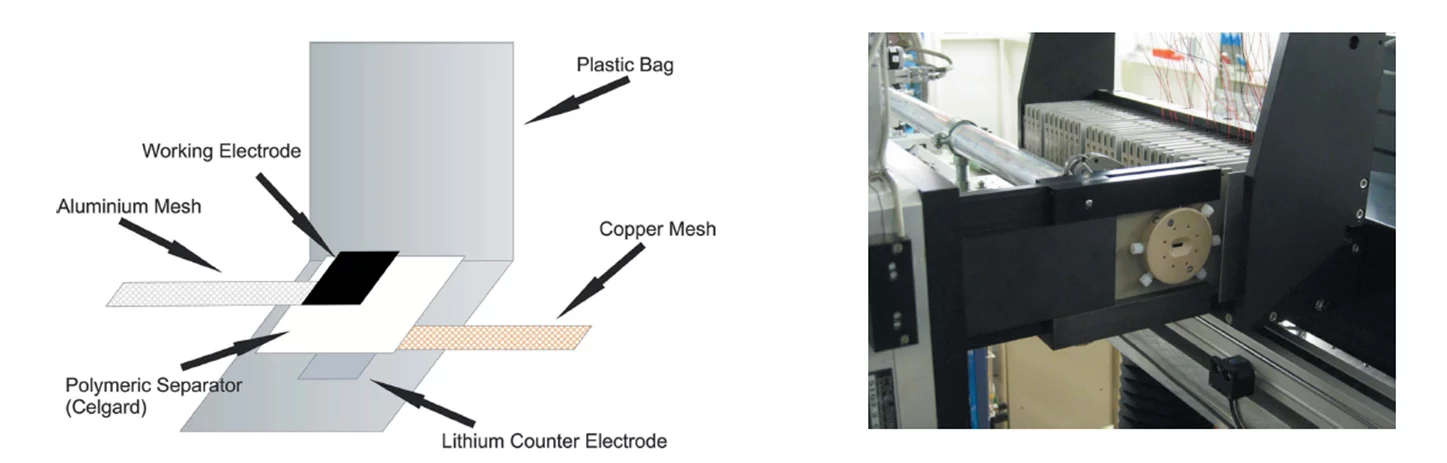
An automatic system that allows for continuous in situ electrochemical X-ray diffraction measurements has been developed and implemented at the MSX04SA beamline at the Swiss Light Source. The system consists of an automatic sample changer, improved ‘coffee bag’ electrochemical cells, and simple control software. The sample changer can sequentially move up to 32 electrochemical cells into the beam. The MYTHEN detector at the beamline enables parallel detection of diffracted X-ray beams and, thus, fast data acquisition, along with a high 2 resolution.
For this purpose we are using the beamline X04 SA at PSI, SLS.
For more information: MS powder beamline
For more information: MS powder beamline
Neutron diffraction at Paul Scherrer Institute
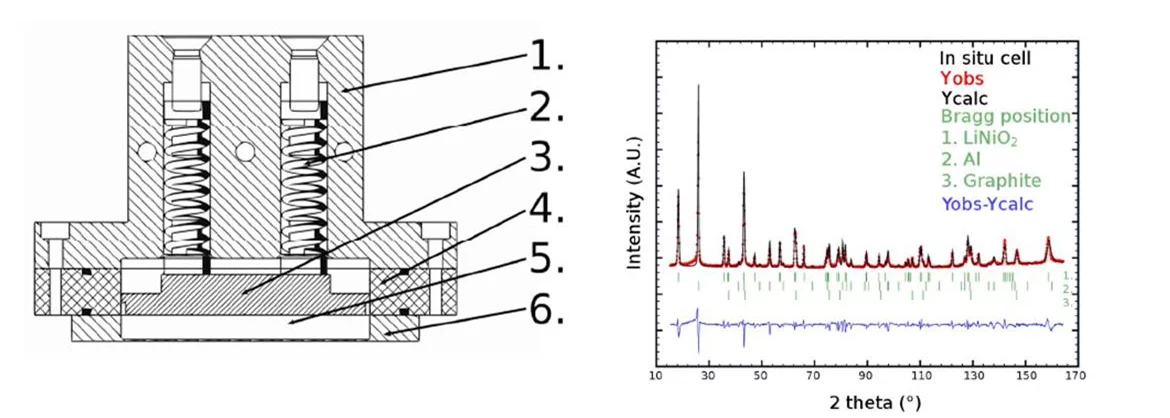
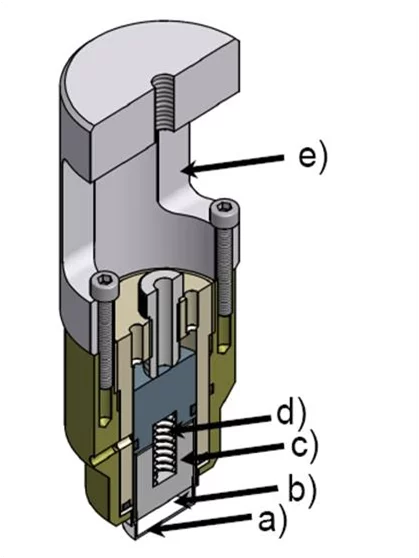
Neutron diffraction is a key challenge for an electrochemical point of view, i.e. it is a powerful, but complicated in situ characterization method. Due to the low interaction of neutrons with matter, a large amount of active material in the sample is needed. Consequently, thick electrodes and high currents are used and that can result in large overpotentials and inhomogeneities in the electrode. Additionally, today's electrolytes are based on organic solvents containing many hydrogen atoms. The substitution of the hydrogen atoms with deuterium is necessary to avoid the strong incoherent scattering and thus optimise the signal to noise ratio of the diffraction experiments. A new cell for in situ neutron diffraction experiments on electrode materials for Li-ion batteries was designed (Figure 2a) to simplify the neutron diffraction method. The suitability of the cell to obtain clear powder diffraction data was tested at the HRPT powder diffractometer at the SINQ neutron source at PSI. A cell was assembled with LiNiO2 as materials for cathode electrode. The resulting diffraction pattern is shown on the right side of the figure.
For this purpose we are using the beamline HRPT at PSI, SINQ.
For more information: SINQ beamline
For more information: SINQ beamline
Neutron diffraction at Institut Laue Langevin (Grenoble, France)
A new cell for in situ neutron diffraction was designed to be used on a more powerful beamline, namely the D20 at the ILL (Grenoble, France). The goals were to improve the time resolution by reducing the counting time for each diffractogram, and to reduce the quantity of active material used in order to get a better electrochemical performance. Another important issue is the geometry of the cell to overcome undesired absorption effects.
The diameter of the sample container is 15mm and its height is 3mm, consequently only 300mg of active material is needed, which makes the electrochemistry much more efficient. Tests were carried out at the D20 beamline and the results are presented in the research highlight (battery materials section).
For more information: ILL D20
For more information: ILL D20
Neutron imaging
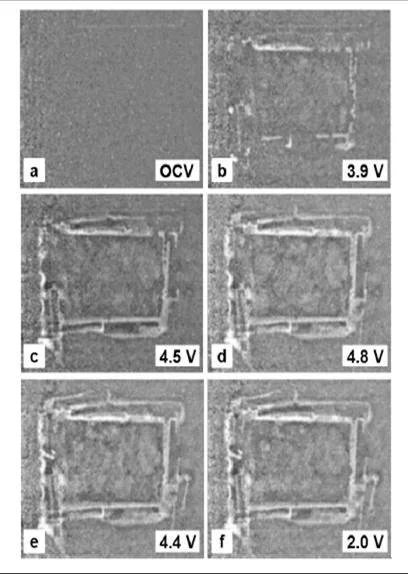
The electrochemical activation of Li2MnO3 can be performed up to 4.4 V and afford an overall specific charge of about 330 Ah/kg. Different analyses methods were applied to study a simultaneous extraction of lithium and oxygen occurring in this potential region. For example, the irreversible oxidation at 4.5 V was further investigated with the differential electrochemical mass spectrometry (DEMS). To visualize the release of gas, neutron imaging at the SINQ source of PSI was applied. The displacement of the liquid electrolyte, observable by neutron imaging (Figure 4), is induced by the gas formation and it is used to indirectly observe the evolution of gas inside a complete Li-ion cell. Moreover, the course of gas release during cycling is presently (Figure 4) observable in situ and provide new informations about the aging processes in lithium-ion batteries.
For more information: ICON
For more information: ICON