Apart from maintaining the VUV beamline at the Swiss Light Source, the Reaction Dynamics Group studies reactions and species relevant to low temperature combustion, catalysis, and atmospheric chemistry. We employ different flavors of coincidence techniques with velocity map imaging and time-of-flight mass spectrometry, together with synchrotron radiation as well as ultrafast lasers. Our goal is to understand chemical reaction dynamics in various energy and time domains.
Scientific Highlights
Editorial Board Appointments for Patrick Hemberger and Christoph Bostedt
The Journal of Physical Chemistry and Photon Science, both ACS journals, expand their Editorial Advisory Boards.
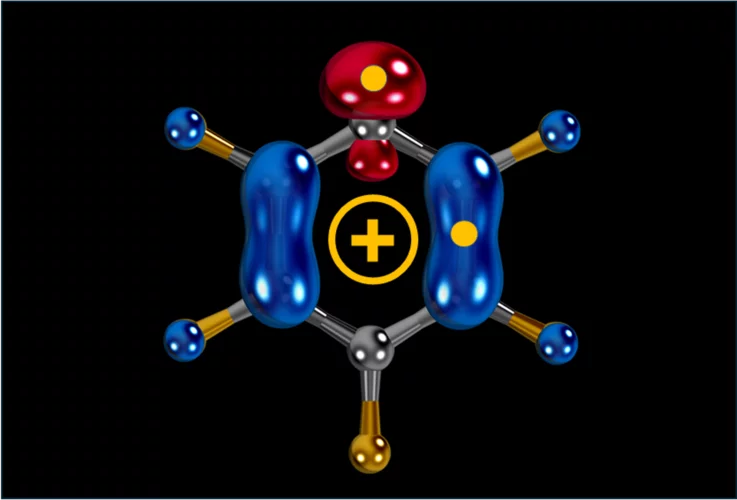
Carbocation, diradical, and superelectrophile in one molecule?
The pentafluorophenyl cation (C₆F₅⁺) breaks these rules with a borderline “crazy” reactivity.
Water gets in shape for VUV absorption
Nanometre‑thin, free‑flowing liquid sheets now let Swiss Light Source users record pristine VUV absorption spectra of water, and soon any solvent.