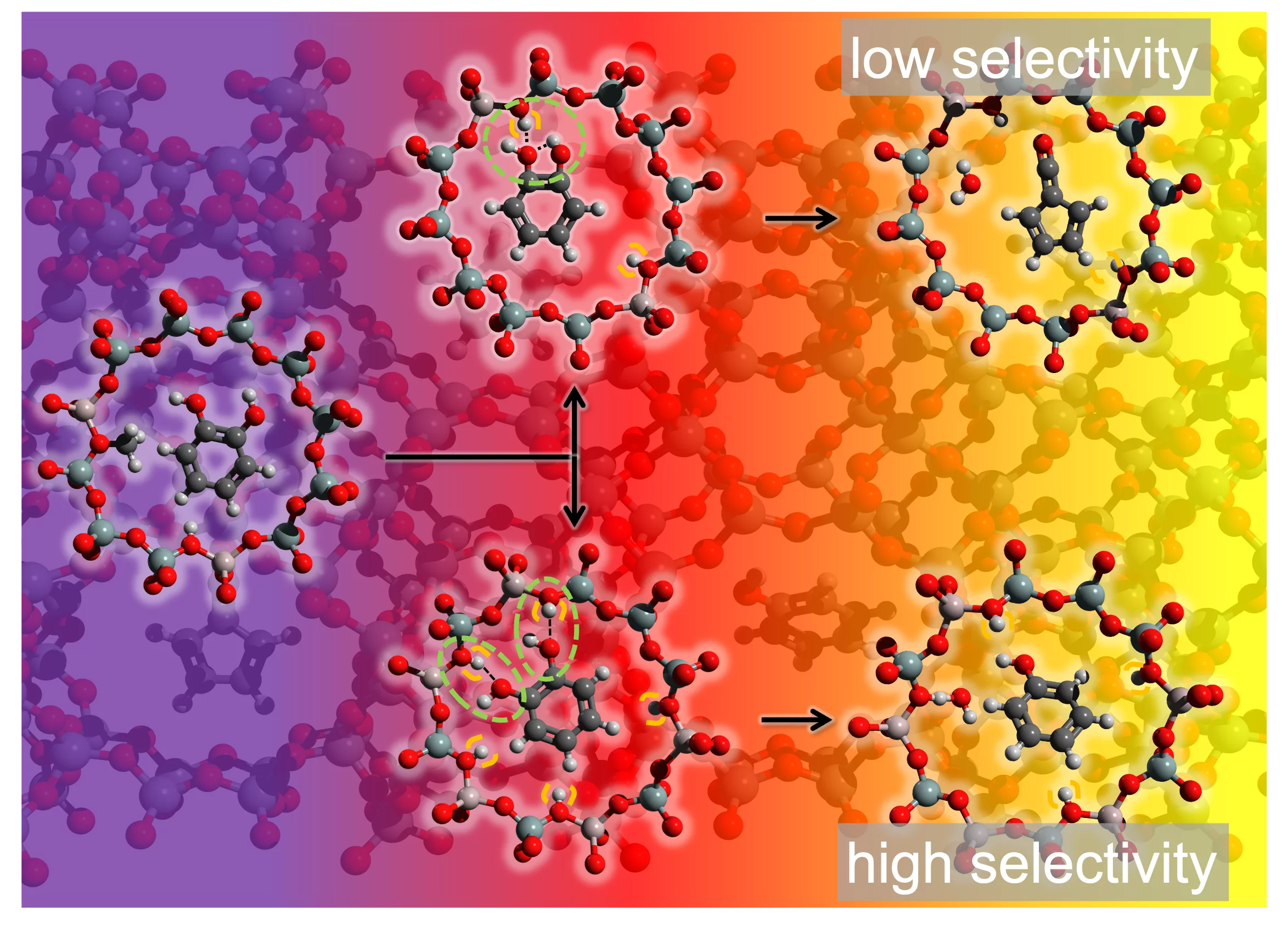
The ultimate goal in heterogeneous catalysis is to design catalysts that enable precise control over selectivity, specifically directing the formation of a desired product. Achieving this objective relies on uncovering both the surface mechanism and the gas-phase chemistry. Understanding the intermediate steps and rate-limiting elementary reactions can assist the catalyst design for enhancing the selectivity through an iterative process.
Researchers from PSI`s Photon Science Division and General Energy Department and ETH Zürich demonstrate how revealing mechanistic insights, via detection of elusive intermediates, enables controlling the selectivity of biomass valorization. This proof-of-principle study, published in Nature Communications, focuses on enhancing the selectivity of the zeolite-catalyzed fast pyrolysis (CFP) of guaiacol (2-methoxyphenol), a lignin model compound, to predominantly yield phenol, an important platform molecule for plastics.
The process starts from the demethylation of guaiacol to catechol (o-benzenediol), as evidenced by elusive methyl radical detection. This reaction then bifurcates into two pathways: one involves catechol dehydration leading to the formation of fulvenone (c-C5H4=C=O), a reactive ketene intermediate, which rapidly produces cyclopentadiene, methylcyclopentadiene, fulvene, and benzene limiting the selectivity (upper trace in Figure). The second route yields phenol almost exclusively through an acid-catalyzed dehydroxylation (lower trace in Figure).
Increasing the Brønsted acid sites density suppresses fulvenone formation due to catechol's bidentate surface coordination on the faujasite catalyst (lower trace in Figure). This essential discovery is facilitated by operando photoelectron photoion coincidence (PEPICO) spectroscopy, developed by the VUV beamline of the Swiss Light Source. PEPICO isomer-selectively detects and quantifies reactive intermediates and products in catalytic reactions and revealed that ketene suppression significantly raises the phenol selectivity. Comprehensive quantum chemical calculations of fulvenone reaction pathways, combined with 29Si NMR-MAS spectroscopy, back the proposed mechanism.
Gaining molecular-level insights into the enhancement of phenol yields in guaiacol CFP is crucial for biomass valorization processes, given that guaiacol is one of the most prevalent building blocks of lignin. Additionally, the potential of operando PEPICO spectroscopy for detecting and quantifying reactive species, extends beyond lignin catalytic pyrolysis or Brønsted acid sites, and has the potential to optimize other heterogeneous catalytic processes such as hydrogenation and syngas- or methanol-to-hydrocarbon reactions.
Article:
Tuning the zeolite acidity enables selectivity control by suppressing ketene formation in lignin catalytic pyrolysis
Nature Communications 2023, 14, 4512.
DOI: https://www.nature.com/articles/s41467-023-40179-z
Contact
Dr. Patrick Hemberger
Reaction Dynamics Group
Paul Scherrer Institut
Telephone: +41 56 310 3236
E-mail: patrick.hemberger@psi.ch
https://www.psi.ch/lsf/patrick-hemberger
Prof. Dr. Jeroen A. van Bokhoven
Laboratory Head LSK
Paul Scherrer Institut
Telephone: +41 56 310 5046
E-mail: jeroen.vanbokhoven@psi.ch
Further Information:
PEPICO | VUV | Paul Scherrer Institut (PSI)