Suchen
LEC Events/Seminars
Die Seminare finden im Raum ODRA/111 jeweils am Mittwoch um 11:00 Uhr statt. Externe Gäste (ausserhalb des PSI) sind willkommen, werden aber gebeten, sich im Labor-Sekretariat bei Frau Cordelia Gloor unter Tel.: +41 56 310 2919 oder per e-mail anzumelden. Lectures take place in room ODRA/111 on every Wednesday at 11:00 am. External guests (from outside PSI) are welcome, but are requested to register with our secretary Cordelia Gloor at phone: +41 56 310 2919 or via e-mail.
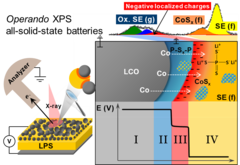
Reactivity and potential profile across the electrified LiCoO2-Li3PS4 interface probed by operando X-ray photoelectron spectroscopy
All-solid-state lithium batteries are a promising alternative for next generation of safe energy storage devices, provided that parasitic side reactions and the resulting hindrances in ionic transport at the electrolyte-electrode interface can be overcome. Motivated by the need for a fundamental understanding of such interface, we present here real-time measurements of the (electro-)chemical reactivity and local surface potential at the electrified interface Li3PS4 and LiCoO2 using operando X-ray photoelectron spectroscopy.
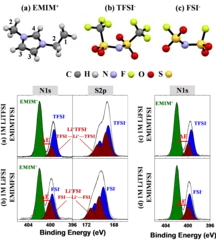
Li-ion solvation in TFSI and FSI - based ionic liquid electrolytes probed by X-ray photoelectron spectroscopy
We demonstrate the capability of conventional laboratory XPS to determine the anions solvation shell of Li+ cation within 1M of LiTFSI and 1M of LiFSI salts dissolved in (EMIM+-FSI-) and (EMIM+-TFSI-) ionic liquids. The binding energy difference between the N1s components originating from the EMIM+ cation and from TFSI- or FSI- anions, solvating the Li+, confirms that both TFSI- and FSI- contribute simultaneously to the Li+ solvation. Additionally, the degradation of the TFSI and FSI -based electrolytes under X-ray exposure is proved.
Integration of Li4Ti5O12 crystalline films on silicon towards high-rate performance lithionic devices
The growth of crystalline Li-based oxide thin films on silicon substrates is essential for the integration of next-generation solid-state lithionic and electronic devices. In this work, we employ a 2 nm γ-Al2O3 buffer layer on Si substrates in order to grow high quality crystalline thin films Li4Ti5O12 (LTO). Long-term galvanostatic cycling of 50 nm LTO demonstrates exceptional electrochemical performance, specific capacity of 175 mAh g-1 and 56 mAh g-1 at 100C and 5000C respectively, with a capacity retention of 91% after 5000 cycles.
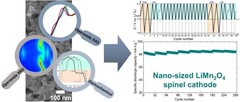
Understanding the (de-)lithiation mechanism of nano-sized LiMn2O4 allows achieving long-term cycling stability
We report an in-depth investigation of the local atomic geometry, electronic and crystallographic structure evolution of nano-sized LiMn2O4 using operando XAS and XRD to shed light on (de-)lithiation mechanism when cycled in wide voltage range of 2.0 to 4.3 V vs Li+/Li. Leveraging on these findings, a novel electrochemical cycling protocol, with periodic deep discharge, yields superior electrochemical performance cycled in the range of 3.3 to 4.3 V exhibiting an excellent structure cyclability and an unprecedented increase in the specific capacity upon long cycling.
Publications 2014
Electrochemistry Laboratory (LEC)
Members of the Electrochemistry Laboratory (Copy)
Electrochemistry Laboratory (LEC)
Publications 2019
Electrochemistry Laboratory (LEC)
Publications 2017
Electrochemistry Laboratory (LEC)
Publications
NRES Publications
Gas Diffusion Layers with Patterned Wettability
During PEFC’s operation the reactant gas is fed to the cell through the gas diffusion layer (GDL) and through the same material the product water is removed. Water accumulation near the catalyst can block the gas access causing an increase of the mass transport losses. On the other hand, PEFC’s work with a solid electrolyte membrane that needs to be hydrated in order to be proton conductive. In order to obtain the maximum performance PEFC’s require a well-balanced water management.
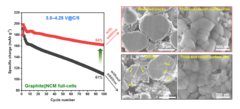
Improved Interfacial Stability of Ni-rich Oxide Full-Cells
PSI researchers have identified a novel electrolyte additive, allowing extended voltage range of Ni-rich oxide full-cells, while keeping excellent performance. The instability of cathode–electrolyte interface causes the structural degradation of cathode active material and the electrolyte consumption, resulting in a rapid capacity fading and shortening battery life-time. The PSI-identified additive help to alleviate these problems and extend battery life-time.
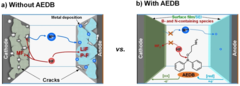
Cross-Talk–Suppressing Electrolyte Additive for Li-ion Batteries
Control of interfacial reactivity at high-voltage is a key to high-energy-density Li-ion batteries. 2-aminoethyldiphenyl borate was investigated as an electrolyte additive to stabilize surface and bulk of both NCM851005 and graphite in the cell with upper cut-off voltage of 4.4 V vs Li+/Li. AEDB almost completely eliminated the “cross-talk” in the cell, by significantly reducing metal leaching from the cathode, preventing their deposition at the anode, and further electrolyte decomposition.
Deciphering the Mechanism of FEC-induced SEI Formation in Li-ion Batteries
Fluoroethylene-carbonate is often referred to as a film-forming electrolyte additive for Li-ion batteries, resulting in high quality Solid–Electrolyte-Interphase on negative electrode, however, the underlying mechanism, even if thought to be known, has been only clarified due to our targeted experimental design, combining systematic electrochemical, chemical and microscopy characterization techniques. We have shown that first the formation of inorganic LiF-rich particles appear and only later the carbonate-rich film is actually formed.