PSI researchers have identified a novel electrolyte additive, allowing extended voltage range of Ni-rich oxide full-cells, while keeping excellent performance. The instability of cathode–electrolyte interface causes the structural degradation of cathode active material and the electrolyte consumption, resulting in a rapid capacity fading and shortening battery life-time. The PSI-identified additive help to alleviate these problems and extend battery life-time.
Ni-rich layer oxides Li[NixCoyMn1−x−y]O2 (x ≥ 0.8, NCMs) are promising advanced cathode materials for high-energy density Li-ion batteries (LIBs) because of their high specific capacity (≥ 200 mAh g−1), as compared to the commercialized cathode materials. The even higher energy density of these materials can be achieved by increasing upper cut-off voltage of the cell, as the energy density depends not only on capacity but also on voltage of the cell. However, the instability of cathode–electrolyte interface, especially under increased voltage range conditions, causes the structural degradation of cathode active material as well as the electrolyte consumption and gas evolution due to oxidative decomposition of electrolyte, resulting in a rapid capacity fading. Thus, improvement of the cathode–electrolyte interphase stability is a key requirement to inhibit Ni-rich active-material structural degradation and enhance its electrochemical performance. Therefore, interfacial stabilization through a novel multifunctional electrolyte additive is of high importance not only for scientific community but also for industry and consumers.
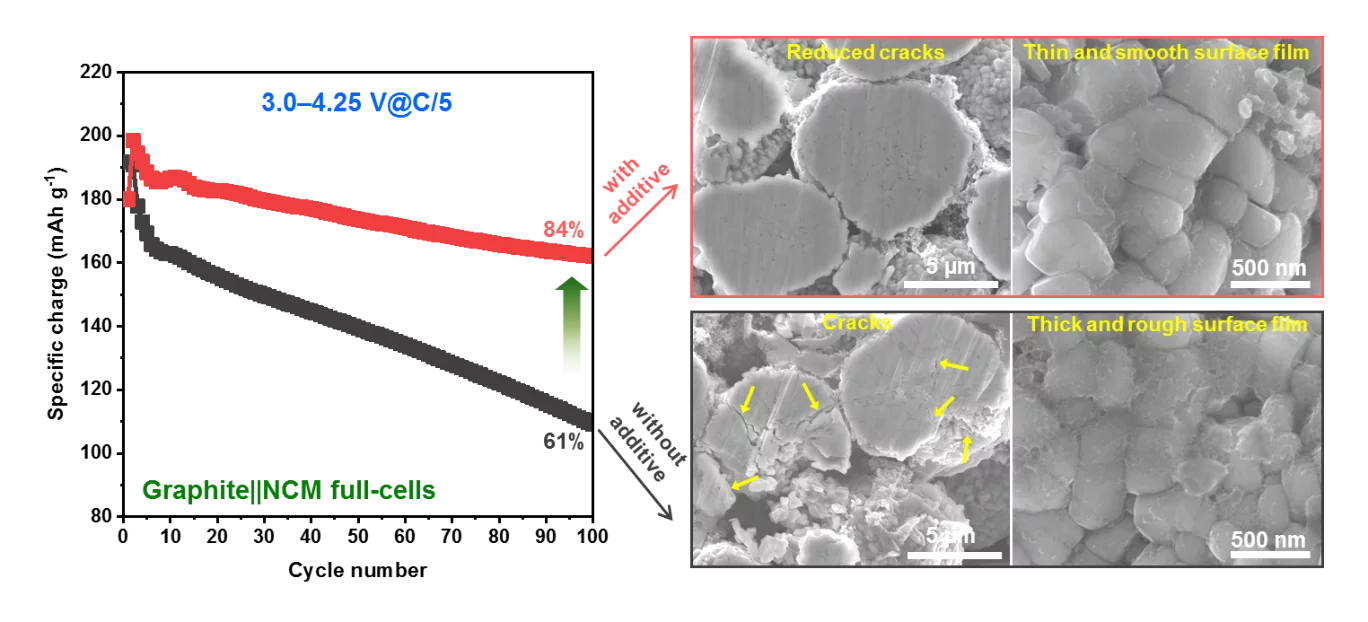
Density-functional-theory calculations, combined with linear sweep voltammetry and cyclic voltammetry suggest that our multifunctional electrolyte additive is oxidized and reduced prior to the solvents, contributing to stable protective surface films on either of the electrodes. Cycling tests confirm that the addition of as little as 1 wt% of multifunctional electrolyte additive significantly reduces the cell polarization and improves the long-term cycling of the cell, which delivers a maximum capacity of 198 mAh g−1, with excellent capacity retention of 84 % after 100 cycles at a cycling rate C/5, in contrast to a rapid performance fade of the cell with conventional electrolyte.
The greatly improved electrochemical performance is attributed to the participation of additive in forming a stable surface protective film at the Ni-rich cathode surface, effectively reducing cracks formation, metal-dissolution, and structural degradation of the cathode upon long-term cycling in elevated voltage window. Moreover, the additive forms a stable solid electrolyte interphase (SEI) layer on the graphite anode, improving cycling performance of the full-cell.
In addition, the identification of this electrolyte additive gives an insight into the design principles of electrolyte additives for high energy-density LIBs and allows to realize stable electrode–electrolyte interfaces.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publication
Multifunctional Electrolyte Additive for Improved Interfacial Stability in Ni-rich Layered Oxide Full-Cells
Hieu Quang Pham, Marta Mirolo, Mohamed Tarik, Mario El Kazzi, Sigita Trabesinger
Energy Storage Materials 33, 216-229 (2020)
DOI: 10.1016/j.ensm.2020.08.026