Recherche
“More objectivity would be helpful”
The current energy debate could do with more facts and less gut feeling – argue Thomas J. Schmidt, renewables expert, and Andreas Pautz, nuclear energy specialist.
Electrocatalysis and Interfaces
The Electrocatalysis and Interfaces Group was established in 2012 combining the electrocatalysis activites of the former Fuel Cell Group and the Interface analytical activities of the former Interface and Capacitor Group. Electrocatalysis is the key topic for electrochemical energy conversion.
Damage-Repair Cycle in Hydrocarbon Based Membranes for Fuel Cells
The development of next generation fuel cell membranes based on aromatic hydrocarbon chemistry calls for a new antioxidant strategy to tackle radical induced membrane degradation. Although damage by radicals cannot be prevented, the formed aromatic intermediates can be repaired by a suitable additive. Fuel cell experiments demonstrate that the approach is viable on the device level and that repair is a catalytic mechanism.
Publications 2015
Electrochemistry Laboratory (LEC)
Publications 2018
Electrochemistry Laboratory (LEC)
Switzerland’s path to the net-zero target
The ETH institutions are pooling their expertise in pursuit of the net-zero target.
Head of Laboratory for Advanced Spectroscopy and X-ray Sources (LSX)
Zukünftige Computerchips mit "elektronischem Blutkreislauf"
Im Rahmen des Sinergia-Programms fördert der Schweizerische Nationalfonds das dreijährige Forschungsvorhaben REPCOOL. Unter der Leitung von IBM Research à Zürich arbeiten in diesem Projekt Wissenschaftler der ETH Zürich, des Paul Scherrer Instituts in Villigen und der Università della Svizzera italiana in Lugano gemeinsam an der Erforschung eines elektronischen Blutkreislaufs für zukünftige 3D-Computerchips. Vom menschlichen Gehirn inspiriert, entwickeln die Forscher ein Mikrokanalsystem mit einer elektrochemischen Flussbatterie, die 3D-Chipstapel gleichzeitig kühlen und mit Energie versorgen. Ultimatives Ziel ist die Entwicklung eines Supercomputers in PC-Grösse.This news release is only available in German.
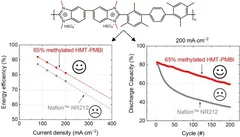
Polybenzimidazole Membrane Design Principles for Vanadium Redox Flow Batteries
Energy storage technologies with long storage duration are essential to stabilize electricity grids with a high share of intermittent renewable power. In a redox flow battery, the electrochemical conversion unit, where the charging and discharging reaction takes place, is spatially separated from the energy storage medium. In the all-vanadium redox flow battery (VRFB), a sulfuric acid aqueous electrolyte with dissolved vanadium ions is used as the storage medium. Vanadium is present in 4 different oxidation states, the redox couple vanadium(II) and (III) on the negative side of the cell, and vanadium(IV) and (V) on the positive side. This allows the battery to be repeatedly charged and discharged. A separator or membrane is used between the negative and positive electrode, which should selectively conduct the ions of the supporting electrolyte and minimize the passage of vanadium ions. Fluorinated membranes, such as Nafion™, are often used for this key component, but these ionomers were not originally developed for this application and therefore have functional shortcomings. Furthermore, the production and use of fluorinated materials is to be severely restricted or even banned in Europe. Therefore, the development of hydrocarbon-based membranes for the VRFB is of great importance. The study reported here focuses on polybenzimidazole polymers and membranes, which could be a promising materials class for next generation flow batteries.
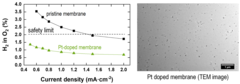
Enabling the use of Thin Membranes in Water Electrolyzers using a Recombination Catalyst
The conversion efficiency for green hydrogen production in a polymer electrolyte water electrolyzer (PEWE) is strongly influenced by the ohmic cell resistance and therefore the thickness of the membrane used. The use of thin membranes (~50 micron or below) is limited by gas crossover of H2 and O2, which can lead to the formation of explosive gas mixtures. The incorporation of a recombination catalyst provides remedy and allows a more dynamic operating mode.
Publications
Thin Films and Interfaces
Five times less platinum: fuel cells could become economically more attractive thanks to novel aerogel catalyst.
Fuel cells that convert hydrogen into power and only produce pure water as a by-product have the potential to lead individual mobility into an environmentally friendly future. The Paul Scherrer Institute (PSI) has been researching and developing such low-temperature polymer electrolyte fuel cells for more than 10 years and initial field tests have already demonstrated the successful use of these fuel cells in cars and buses. However, further research is still required to improve the durability and economic viability of the technology. An international team of researchers involving the PSI has now manufactured and characterised a novel nanomaterial that could vastly increase the efficiency and shelf-life of these fuel cells à as well as reduce material costs.
Ice in fuel cells imaged directly for the first time
Researchers from the Paul Scherrer Institute (PSI) have succeeded in imaging the distribution of frozen and liquid water in a hydrogen fuel cell directly for the first time. They applied a new imaging technique that uses successively two beams with different neutron energies to distinguish between areas with liquid water and those with ice extremely reliably. The method therefore opens up the prospect of studying one of the main problems of using fuel cells to power vehicles: ice can clog the pores in the fuel cells and affect their performance. The PSI scientists’ results will be published in the journal Physical Review Letters on 16 June 2014.
List of papers describing details of the beamline
Please cite the corresponding beamline paper in your publications. Contact the beamline scientist if you are not sure which paper you should cite.
Collaborations
We are working together in our actual (and past ) projects with various national and international collaboration partners.