The conversion efficiency for green hydrogen production in a polymer electrolyte water electrolyzer (PEWE) is strongly influenced by the ohmic cell resistance and therefore the thickness of the membrane used. The use of thin membranes (~50 micron or below) is limited by gas crossover of H2 and O2, which can lead to the formation of explosive gas mixtures. The incorporation of a recombination catalyst provides remedy and allows a more dynamic operating mode.
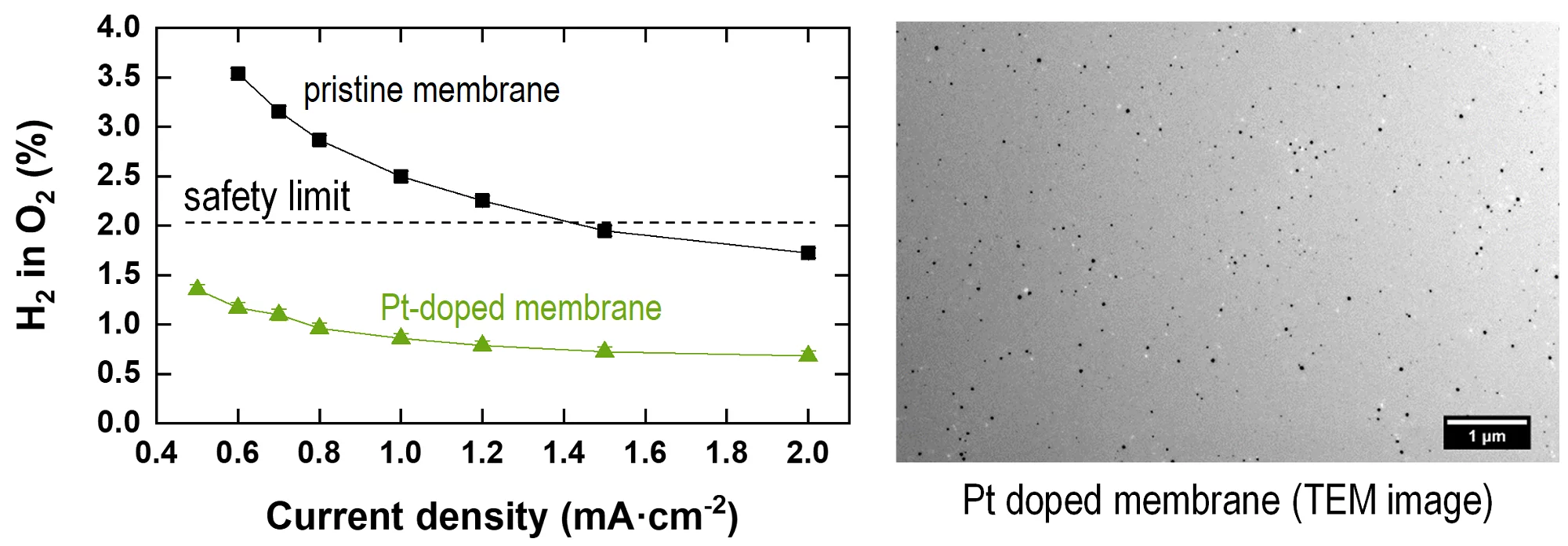
State of the art polymer electrolyte water electrolyzers typically use a perfluorinated proton exchange membrane of the Nafion type with a thickness of 150 to 200 micron. Such thick membranes lead to a large ohmic drop in the cell, limiting the conversion efficiency for hydrogen production. The ohmic resistance can be significantly reduced with the use of thinner membranes, such as Nafion 212 with a dry thickness of 60 micron. This, however, leads to an increase in the H2 and O2 crossover through the membrane. In particular, H2 has a high diffusivity in Nafion, and the accumulation of hydrogen in the oxygen product stream can lead to the formation of an explosive gas mixture (the safety limit of H2 in O2 is 2%). An approach to mitigate gas crossover to the opposite compartment of the cell is to introduce a so-called recombination catalyst into the membrane. These are platinum (or palladium) nanoparticles, which are deposited in the membrane. The dissolved H2 and O2 in the membrane react (‘recombine’) on the surface of the Pt-catalyst to water, thereby reducing the rate of gas crossover. In the Figure, left, the content of H2 in O2, measured at the gas outlet, is shown as a function of current density for an unmodified (pristine) membrane and a Pt-doped membrane during cell operation. With the pristine membrane, the safety limit of 2% is exceeded at a current density below 1.4 A/cm2. Above that, the produced O2 provides sufficient dilution of the crossover H2 for the concentration to be below 2 %. With the Pt-doped membrane the H2 crossover is much lower and the cell can be operated down to low current densities, which greatly enhances the dynamic operation required if the electrolyzer is connected to a dynamic power supply. The operating current range is of particular importance if the electrolysis system is used for grid stabilization services. The cell performance is not significantly affected by the presence of the Pt-nanoparticles in the membrane, the cell voltage is only 32 mV higher at 2 A/cm2 for the Pt-doped membrane compared to the pristine membrane. We investigated different methods of Pt-doping of membranes. Initially, Pt[(NH3)4]2+ was introduced into the Nafion membrane by partial ion-exchange. Then, in a method adopted from the literature, the Pt(II) doped membrane was immersed into N2H4 solution to reduce the Pt-ions to metallic Pt clusters. In a novel approach (Patent application EP19175071.0,2019) hydrogen was used as a reducing agent, which led to a homogeneous dispersion of Pt-particles in the membrane with a size in the range of a few tens of nanometers (Figure, right).
Contact
PD Dr. Lorenz Gubler
Head Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villligen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publications
Communication – Pt-Doped Thin Membranes for Gas Crossover Suppression in Polymer Electrolyte Water Electrolysis
Steffen Garbe, Ugljesa Babic, Elisabeth Nilsson, Thomas J. Schmidt, Lorenz Gubler
J. Electrochem. Soc. 166 (13), F873-F875 (2019).
DOI: 10.1149/2.0111913jes
Comparison of Pt-Doped Membranes for Gas Crossover Suppression in Polymer Electrolyte Water Electrolysis
Steffen Garbe, Erik Samuelsson, Thomas J. Schmidt, Lorenz Gubler
J. Electrochem. Soc. 168, 104502 (2021).
DOI: 0.1149/1945-7111/ac2925
Acknowledgement
Swiss Federal Office of Energy (grant number SI/501603).
Patent
Method for Preparing a Polymer Membrane for a Polymer Electrolyte Water Electrolyser
Steffen Garbe, Ugljesa Babic, L. Gubler, Thomas J. Schmidt
European Patent Application EP19175071.0 (2019).