The development of next generation fuel cell membranes based on aromatic hydrocarbon chemistry calls for a new antioxidant strategy to tackle radical induced membrane degradation. Although damage by radicals cannot be prevented, the formed aromatic intermediates can be repaired by a suitable additive. Fuel cell experiments demonstrate that the approach is viable on the device level and that repair is a catalytic mechanism.
Today’s polymer electrolyte fuel cells (PEFCs), used for example in the Toyota Mirai, contain perfluoroalkylsulfonic acid (PFSA) membranes, which show high chemical stability and proton conductivity. These fluorinated materials have been around for over 50 years and have seen many technological advances, such as thin reinforced membranes, end-group stabilization, short side-chain, and the addition of radical scavengers. Nevertheless, PFSA membranes have some inherent disadvantages in fuel cell applications, in particular a low glass transition temperature of ~110°C, which limits higher temperature operation, and high gas permeability.
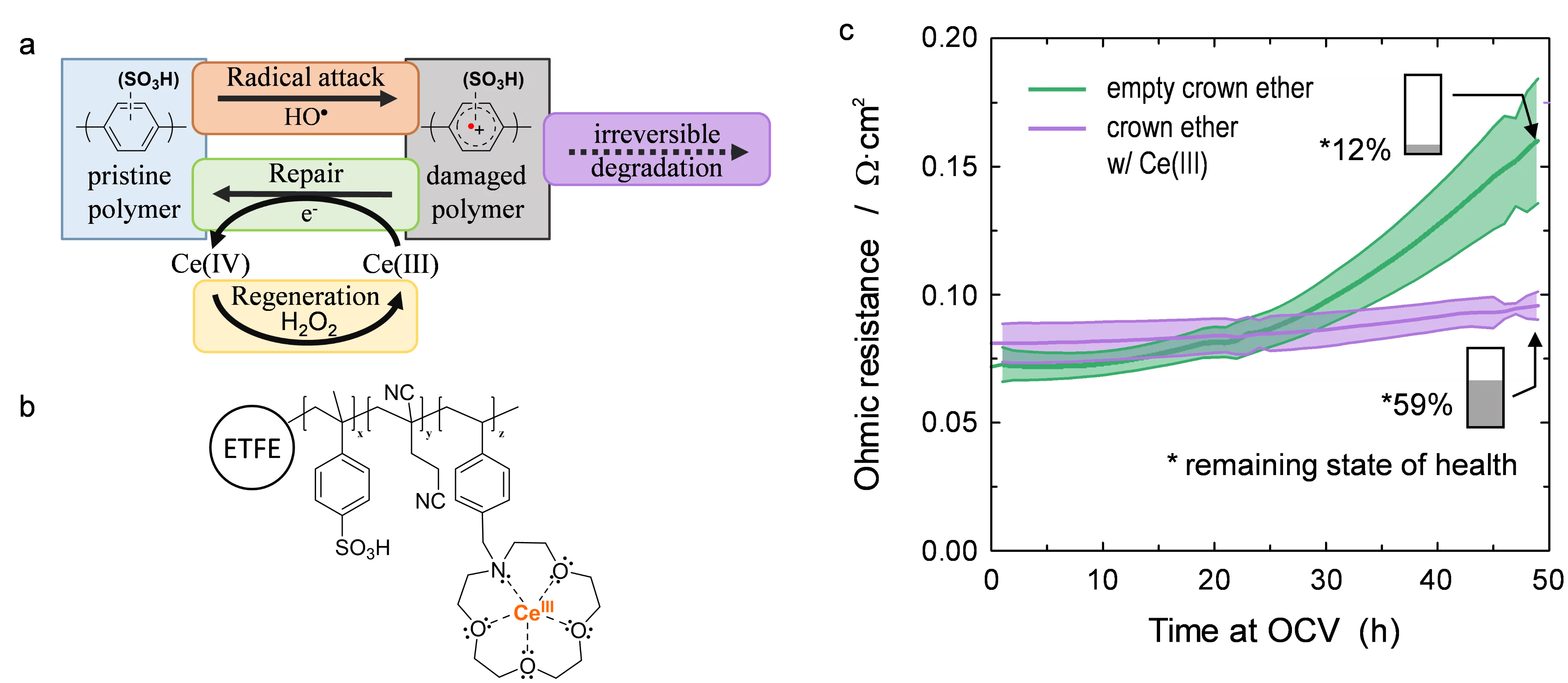
Hydrocarbon-based polyaromatic ionomers are therefore of interest for next-generation proton conducting membranes in the PEFC. Their most critical shortcoming is the presence of aromatic groups contained within the polymer, which make these materials susceptible to HO· radical induced degradation reactions. In PFSA membranes, the lifetime of HO· is in the range of microseconds, which allows the use of radical scavengers to mitigate HO· attack on the polymer and subsequent degradation. Owing to the much faster rate of reaction of HO· with aromatic compounds, Ar, radical scavenging is ineffective and HO· attack is virtually unavoidable. It is, however, conceivable that the intermediates formed upon radical attack are sufficiently long-lived to allow repair by a suitable antioxidant. In particular, cation radicals, Ar·+, have shown to be such intermediates. In pulse radiolysis studies, we have shown that they can be repaired by Ce(III) ions to restore the parent compound, Ar. The formed Ce(IV) reacts with H2O2 present in the fuel cell in the concentration range of millimolar, whereby Ce(III) is restored. Therefore, the repair of cation radicals by Ce(III) may be a catalytic reaction. Consequently, the rate of irreversible degradation of the membrane is significantly reduced (Figure, a).
The concept of membrane stabilization by addition of Ce(III) was subsequently investigated in the fuel cell. We prepared radiation grafted membranes containing a crown ether pendant group (Figure, b), which served to complex the Ce(III) ions to prevent their loss by leaching. An accelerated stress test was used to assess the rate of chemical membrane degradation in the cell, with and without complexed cerium. The measured ohmic resistance of the cell (Figure, c) is an indication of the state of health of the membrane. The much lower increase in the presence of cerium suggests that chemical degradation is mitigated, which is confirmed by the remaining ion exchange capacity (IEC) at the end of test after 50 h. From the chemical degradation studies of PFSA membranes, Ce(III) is known to act as a radical scavenger. However, in the presence of aromatic polymer constituents, the reaction of Ce(III) with HO· is too slow to have a notable scavenging yield. Therefore, it is likely that Ce(III) serves as a repair agent, which acts on intermediates with a lifetime in the range of microseconds, to reduce irreversible polymer chain breakdown. Based on post-test analysis of membranes, we estimated that each cerium-ion was involved in ~25 repair reactions, which supports the notion of a catalytic repair mechanism.
Contact
PD Dr. Lorenz Gubler
Head Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publications
Repair of Aromatic Hydrocarbon-Based Membranes tested under Accelerated Fuel Cell Conditions
Tym de Wild, Tamas Nemeth, Pascal Becker, Detlef Günther, Thomas Nauser, Thomas J. Schmidt, Lorenz Gubler
J. Power Sources 560, 232525 (2023).
DOI: 10.1016/j.jpowsour.2022.232525
Chemical Stability Enhancement of Aromatic Proton Exchange Membranes using a Damage Repair Mechanism
Tym de Wild, Tamas Nemeth, Thomas Nauser, Thomas J. Schmidt, Lorenz Gubler
ECS Transactions 109(9), 317-325 (2022).
DOI: 10.1149/10909.0317ecst
Moderation of Oxidative Damage on Aromatic Hydrocarbon-Based Polymers
Tamas Nemeth, Tym de Wild, Lorenz Gubler, Thomas Nauser
J. Electrochem. Soc. 169, 054529 (2022).
DOI: 10.1149/1945-7111/ac6f85
Impact of Substitution on Reactions and Stability of One-Electron Oxidised Phenyl Sulfonates in Aqueous Solution
Tamas Nemeth, Tym de Wild, Lorenz Gubler, Thomas Nauser
Phys. Chem. Chem. Phys. 24, 895-901 (2022).
DOI: 10.1039/d1cp04518k
Possible Repair Mechanism for Hydrocarbon-Based Ionomers Following Damage by Radical Attack
Tym de Wild, Tamas Nemeth, Tom M. Nolte, Thomas J. Schmidt, Thomas Nauser, Lorenz Gubler
J. Electrochem. Soc. 168, 054514 (2021).
DOI: 10.1149/1945-7111/abf9be
Acknowledgement
Swiss National Science Foundation (grant number 175493).