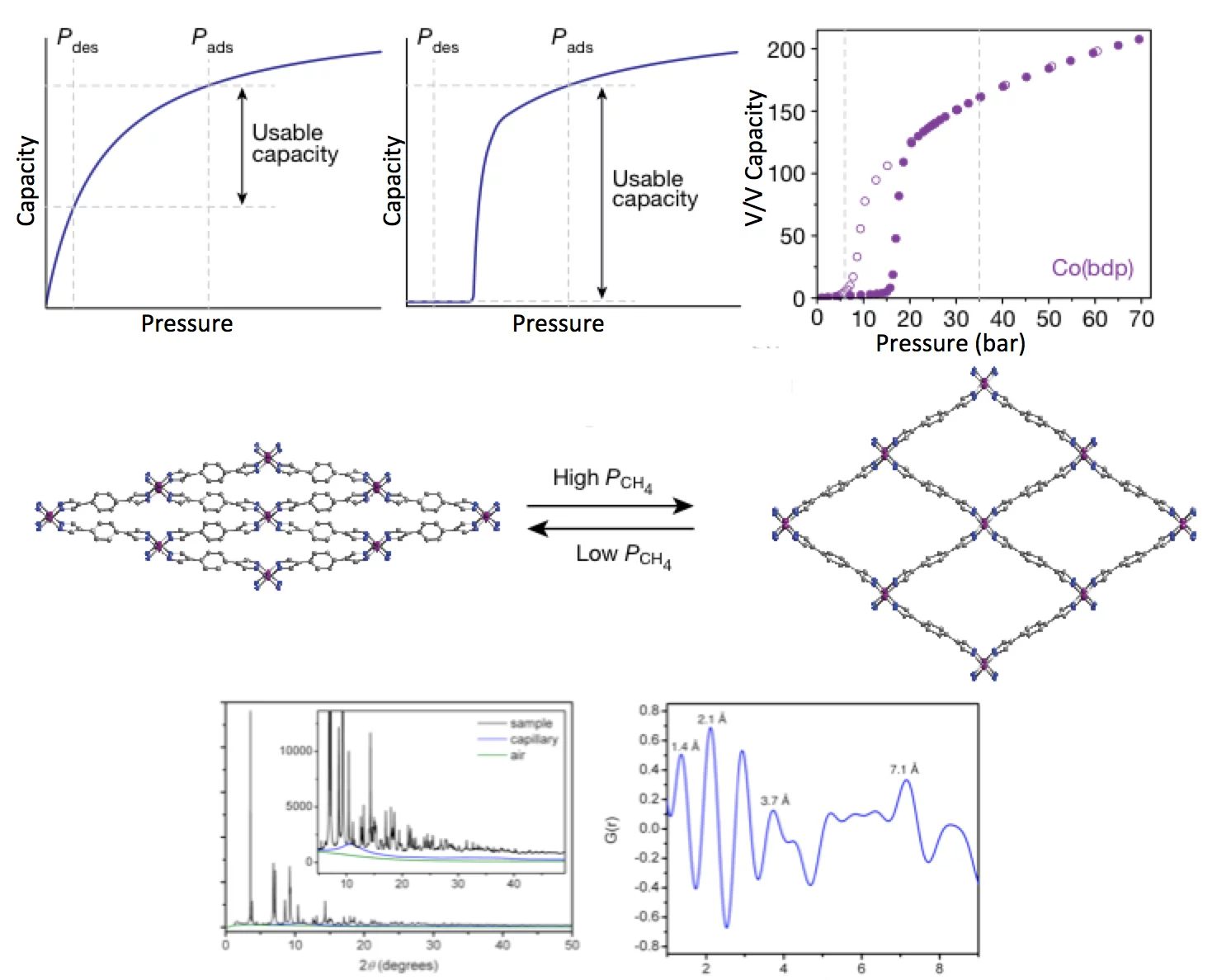
As a cleaner, cheaper, and more globally evenly distributed fuel, natural gas has considerable environmental, economic, and political advantages over petroleum as a source of energy for the transportation sector. Despite these benefits, its low volumetric energy density at ambient temperature and moderate pressure presents substantial challenges, particularly for light-duty vehicles with little space available for on-board fuel storage. Adsorbed natural gas systems have the potential to store high densities of methane (CH4, the principal component of natural gas) within a porous material at ambient temperature and moderate pressures. Activated carbons, zeolites, and metal–organic frameworks have been investigated extensively for CH4 storage, showing practical challenges involved in designing systems with high capacities and in managing the thermal fluctuations associated with adsorbing and desorbing gas from the adsorbent. Here, we demonstrate that the flexible compounds Fe(bdp) and Co(bdp) (bdp = 1,4-benzene dipyrazolate) undergo a structural phase transition in response to specific CH4 pressures, resulting in adsorption and desorption isotherms that feature a sharp step. Such behaviour enables greater storage capacities than have been achieved for classical adsorbents, while also greatly reducing the amount of heat released or absorbed during adsorption and desorption, thanks to the mitigating effect of the phase transition enthalpy. The pressure and energy associated with the phase transition can be tuned either chemically or by application of mechanical pressure. This work uses results from several experiments, including Total Scattering XRPD at the X04SA-Materials Science beamline of the SLS.
Full-text link
Full-text link
Original Publication
Methane storage in flexible metal–organic frameworks with intrinsic thermal managementJ. A. Mason, Julia Oktawiec, M. K. Taylor, M. R. Hudson, Julien Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi, J. R. Long
Nature (2015), Published online, 26 October 2015 DOI: 10.1038/nature15732
Contact
Dr Antonio CervellinoLaboratory for Synchrotron Radiation — Condensed Matter Physics
Swiss Light Source, Paul Scherrer Institute
5232 Villigen PSI, Switzerland
Phone: +41 56 310 4611, e-mail: antonio.cervellino@psi.ch