Strontium titanate, SrTiO3, is an important material for the realization of next-generation electronic devices. A famous example is the interface of LaAlO3 grown on SrTiO3, which is metallic and magnetic at its interface, even though the individual compounds are insulating and nonmagnetic in bulk form. The physics behind how novel interface states form on SrTiO3 - and how they become endowed with such surprising properties - is not well understood. Researchers recently discovered that SrTiO3 can even host a metallic state at its surface (in other words, its interface with vacuum), which opened a new route to studying how conducting electrons behave when they are confined to a small surface or interface region of the material.
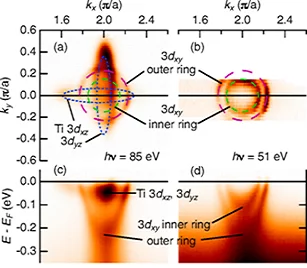
A collaboration led by scientists at PSI used detailed high-resolution angle-resolved photoemission spectroscopy (ARPES) at the Surface/Interface Spectroscopy (SIS) beamline in order to obtain the clearest view to date of the electronic structure of the metallic surface state on SrTiO3. ARPES is a uniquely powerful technique for probing and visualizing electrons' energy and momentum states, which determine a material's most fundamental electronic properties. The experiments revealed that the surface's conducting electrons come in two basic forms that behave in drastically different ways. Those that are associated with titanium 3dxy orbitals tend to be highly confined to a narrow surface region. Others associated with 3dxz and 3dyz orbitals occupy different energy levels from the 3dxy electrons and penetrate several layers or more into the subsurface region. The ARPES data show that, in addition to their spatial segregation, these two types of electrons have different conducting properties - not only relative to each other, but also relative to how they would be expected to behave in the bulk of SrTiO3. The study also found clues to how the surface state forms, including the relative influence of surface defects (against which the conducting electrons are highly robust) and other effects - perhaps structural - that more significantly alter the character of the valence electrons.
A collaboration led by scientists at PSI used detailed high-resolution angle-resolved photoemission spectroscopy (ARPES) at the Surface/Interface Spectroscopy (SIS) beamline in order to obtain the clearest view to date of the electronic structure of the metallic surface state on SrTiO3. ARPES is a uniquely powerful technique for probing and visualizing electrons' energy and momentum states, which determine a material's most fundamental electronic properties. The experiments revealed that the surface's conducting electrons come in two basic forms that behave in drastically different ways. Those that are associated with titanium 3dxy orbitals tend to be highly confined to a narrow surface region. Others associated with 3dxz and 3dyz orbitals occupy different energy levels from the 3dxy electrons and penetrate several layers or more into the subsurface region. The ARPES data show that, in addition to their spatial segregation, these two types of electrons have different conducting properties - not only relative to each other, but also relative to how they would be expected to behave in the bulk of SrTiO3. The study also found clues to how the surface state forms, including the relative influence of surface defects (against which the conducting electrons are highly robust) and other effects - perhaps structural - that more significantly alter the character of the valence electrons.
ORIGINAL PUBLICATION
Mixed Dimensionality of Confined Conducting Electrons in the Surface Region of SrTiO3N. C. Plumb, M. Salluzzo, E. Razzoli, M. Månsson, M. Falub, J. Krempasky, C. E. Matt, J. Chang, M. Schulte, J. Braun, H. Ebert, J. Minár, B. Delley, K.-J. Zhou, T. Schmitt, M. Shi, J. Mesot, L. Patthey, and M. Radović,
Phys. Rev. Lett. 113, 086801 (2014). Published 18 August, 2014.
DOI: 10.1103/PhysRevLett.113.086801
CONTACT
Dr. Nicholas PlumbPaul Scherrer Institut, Switzerland
E-Mail: nicholas.plumb@psi.ch
Dr. Milan Radović
Paul Scherrer Institut, Switzerland
E-Mail: milan.radovic@psi.ch