Intermetallic compounds are optimum alternatives to the largely used molecular plated oxidic samples in various nuclear physic and radiochemistry studies, such as irradiation targets for production of superheavy elements and for nuclear data determination. They have, in fact, better chemical and mechanical stability, higher thermal and electrical conductivity, which make them ideal targets. This work aimed to produce thin intermetallic samples of lanthanides (Ln) and platinoid metals (Pts) via Coupled Reduction (CR) [1], specifically, thermal treatment at 1100 °C under H2 atmosphere. Lanthanide species, were previously molecular plated (MP) on a thin Pts backings, with a concentration of 0.8 at.%, atomic ratio Ln/Pts.
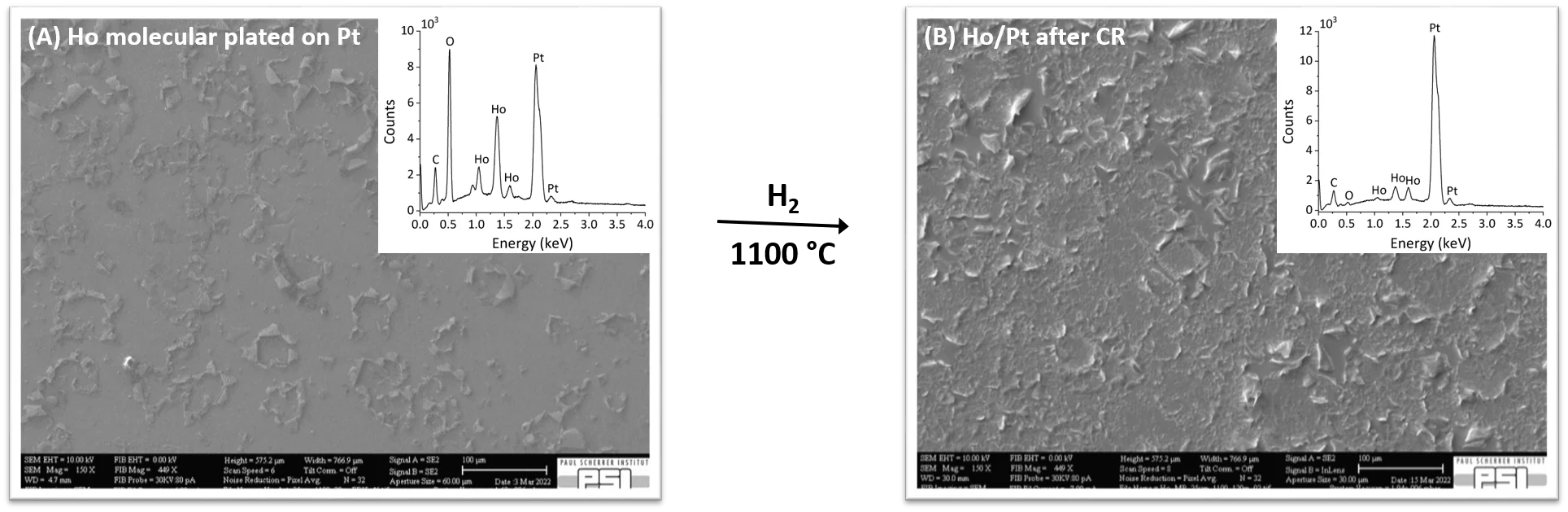
Fig. 1 (A) shows the Scanning Electron Microscope (SEM) picture of a sample obtained by MP of Ho onto Pt backing. It is possible to notice the formation of a not homogeneous thin film with presence of peeled off area. The inset, Energy Dispersive X-Ray Spectroscopy (EDX), shows the chemical composition of the film, characterized by Ho, O, C, and Pt, indicating the chemical speciation of the Ho as carboxylate, oxide or hydroxide. Fig. 1 (B) shows the SEM picture of the same sample after CR at 1100 °C under H2 atmosphere. It is clear that Ho is still partially present as a thin film with a morphology similar to the sample before CR. However, the relative EDX indicates the absence of oxygen, thus the reduction of Ho to its elemental state.
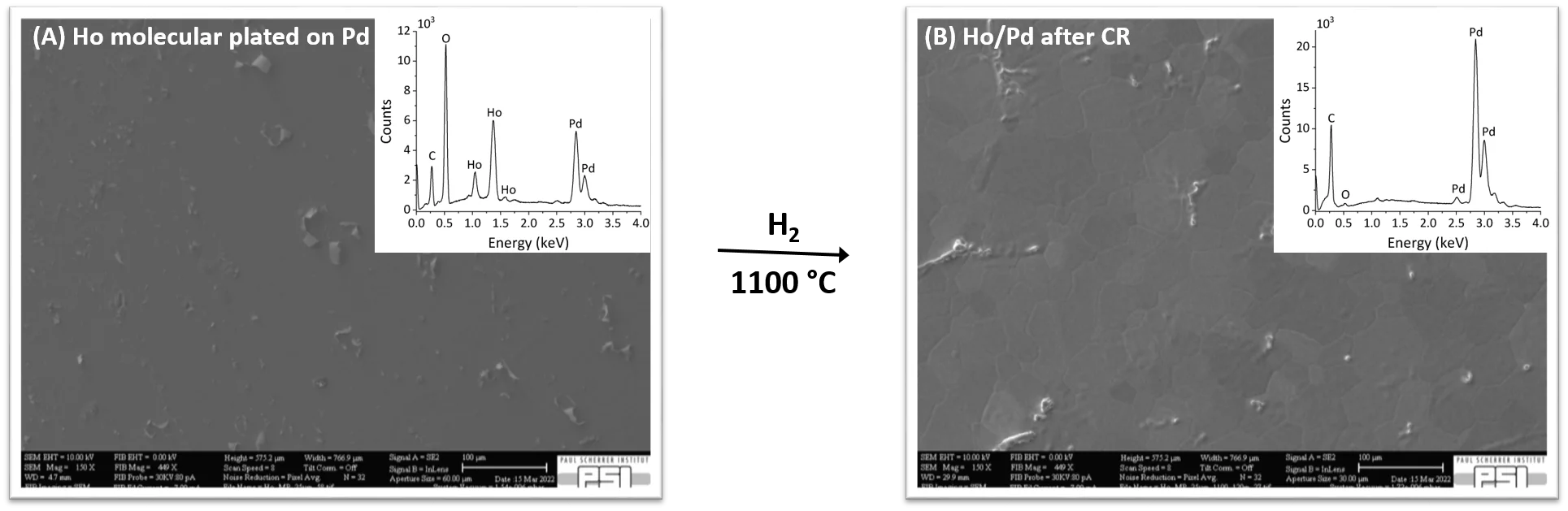
The SEM image of Ho molecular plated on Pd, Fig. 2 (A), shows a more homogeneous surface respect to the Ho molecular plated on Pt, but with similar chemical composition, while different result is obtained by CR. The EDX, inset Fig.2 (B), indicates that Ho is not anymore accumulated on the surface, thus it has reacted and diffused into the Pd matrix, forming a new crystal structure, Fig. 2 (B), i.e. the Ho/Pd intermetallic.
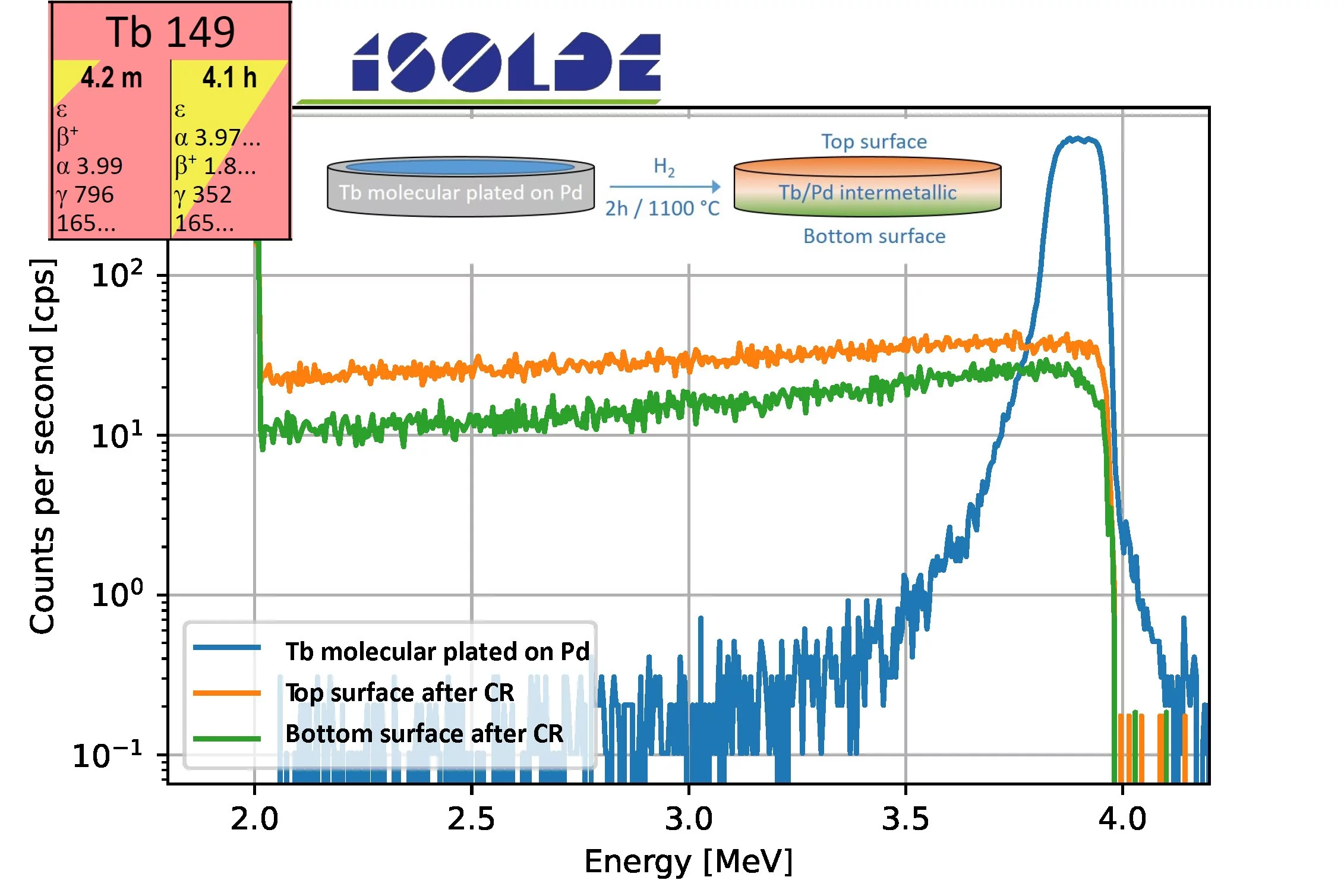
The formation of Lns/Pd intermetallic via CR was further investigated with the couple Tb/Pd using the medically promising 149Tb radionuclide as alpha emitter tracer, provided by CERN ISOLDE. Fig. 3 shows the comparison of the alpha spectra before and after the reduction (from both sides of the sample). In particular, the blue curve, indicates a surface distribution of Tb, which is the result of the molecular plating, the orange and green curves are the spectra of the top and bottom of the sample after reduction (the inset visually illustrates the process). The result indicates an almost homogeneous distribution of Tb in the Pd after the coupled reduction, confirming the previous results, i.e. the formation of an intermetallic compound.
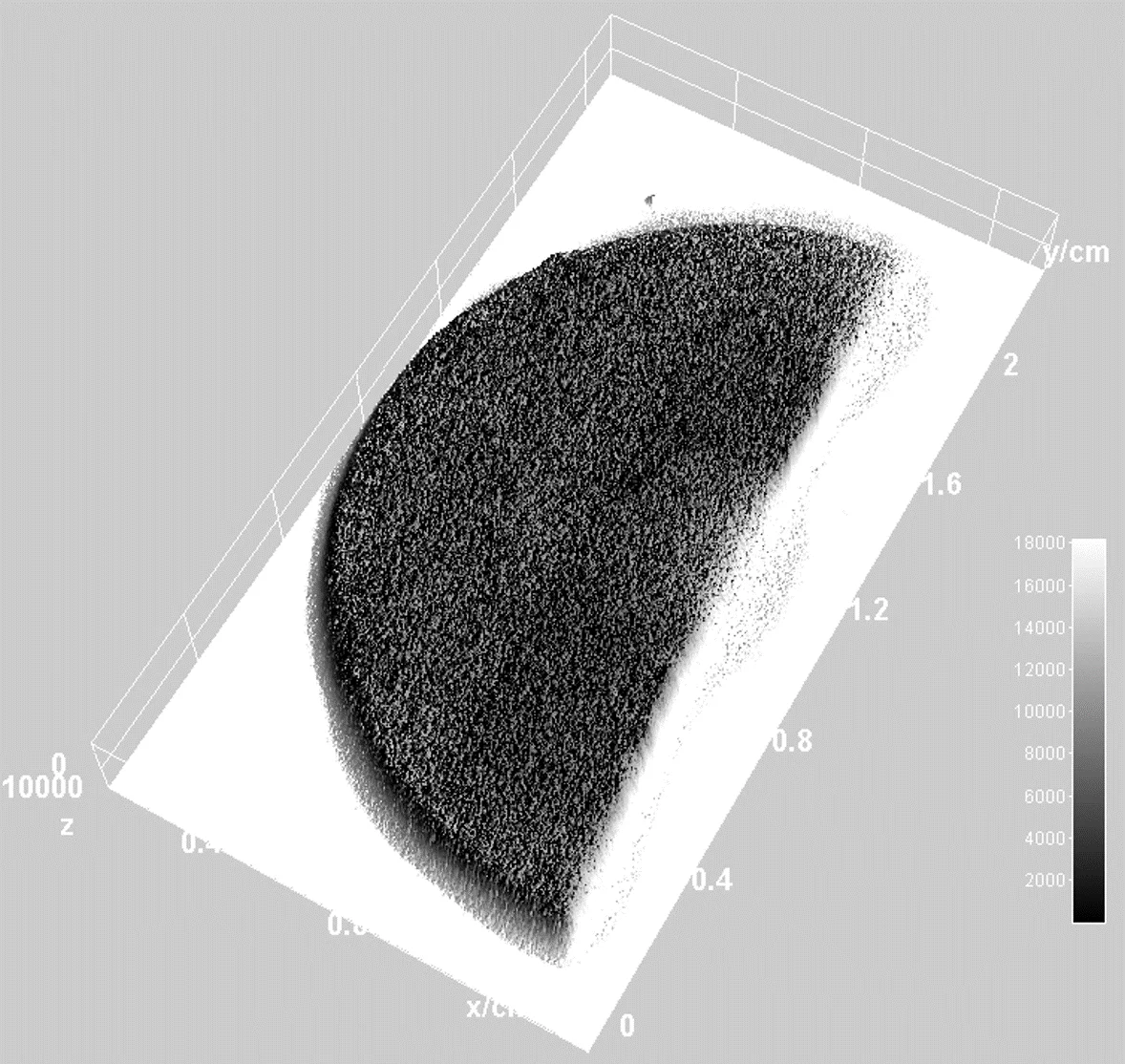
The distribution of 149Tb, obtained from CERN ISOLDE and chemically purified at PSI, into Pd was also investigated via Radiographic Imaging. The resulting three-dimensional radiographic image, Fig. 4, confirms the homogeneous distribution of Tb into the Pd bulk.
Concluding, we have demonstrated that, at defined thermodynamic conditions (temperature, pressure and concentration) the Coupled Reduction method yields a lanthanide thin film on the platinum surface. On the other hand, the formation of an intermetallic compound was obtained when using Pd as backing - as such a sample with the thickness of the used backing material.
Thin films of metallic Lns can be used in different application, such as:
- sources of radiolanthanides for the measurement of Auger electron energies and branching ratios, which are essential nuclear data in dosimetry when using these isotopes for cancer therapy.
- model for the production of metallic actinides films suitable as targets for superheavy elements experiments
Intermetallic Ho/Pd will be used for producing a source of 163Ho for the HOLMEs experiment, which aims to experimentally measure the neutrino mass.
Author
Dr. Emilio Andrea Maugeri
+41 56 310 24 07
emilio-andrea.maugeri@psi.ch
Laboratory of Radiochemistry, LRC
Isotopes and Target Chemistry Group
Original Publication
...................