Cement is widely used as matrix and backfill for low and intermediate level waste. Calcium-Aluminum-Silicate Hydrates (C-A-S-H) are the most important binding phases in cement. They are also responsible for the initial entrapment of radionuclides via sorption or solid solution formation mechanisms. Therefore, the thermodynamic modelling of C-A-S-H stability, solubility and interaction with radionuclides in cement porewater is crucial for understanding hydration, blending, degradation of cement-based materials and for the performance assessment of cementitious repositories.
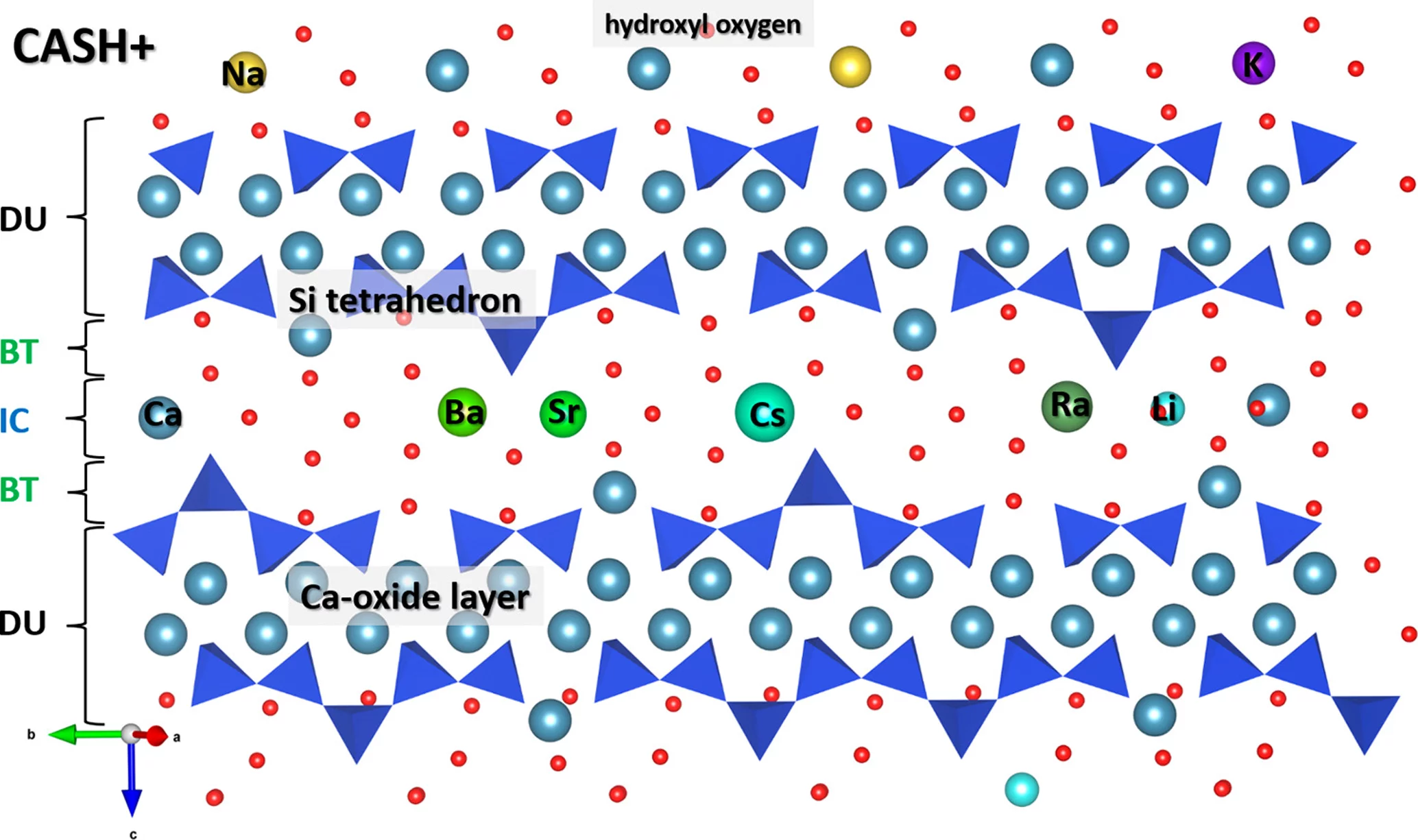
Thermodynamic description of CASH is intrinsically difficult due to “gel-like” nanoparticulate state of this phase. Spectroscopic studies and molecular simulations reveal that the short-range order in C-A-S-H can be described using a lattice defects model based the structure of crystalline mineral tobermorite. Cations in C-A-S-H occupy specific structural positions, which can be associated with analogous structural sites in tobermorite crystal lattice.
With this structural information and the experimental data from numerous solubility studies, an incrementally extendable solid solution model for C-A-S-H was developed using the so-called sub-lattice model with real and virtual end members. The use of structural constraints enhances the thermodynamic consistency and ensures the theory-based accurate predictions of CASH solid solution properties in a multicomponent system.
Relative to older models, the latest improvement consists in a more accurate description of C-A-S-H stability, solubility and cation uptake from aqueous solutions. The CASH+ is the first incrementally extendable model of calcium silicate hydrate, which means that endmembers for the new cation can be added and their thermodynamic properties fitted or predicted without any re-fitting of properties of the previously available endmembers.
The CASH+ solid solution model is actually a thermodynamic database for endmembers and site interaction parameters, which grows with each next cation endmembers set added.
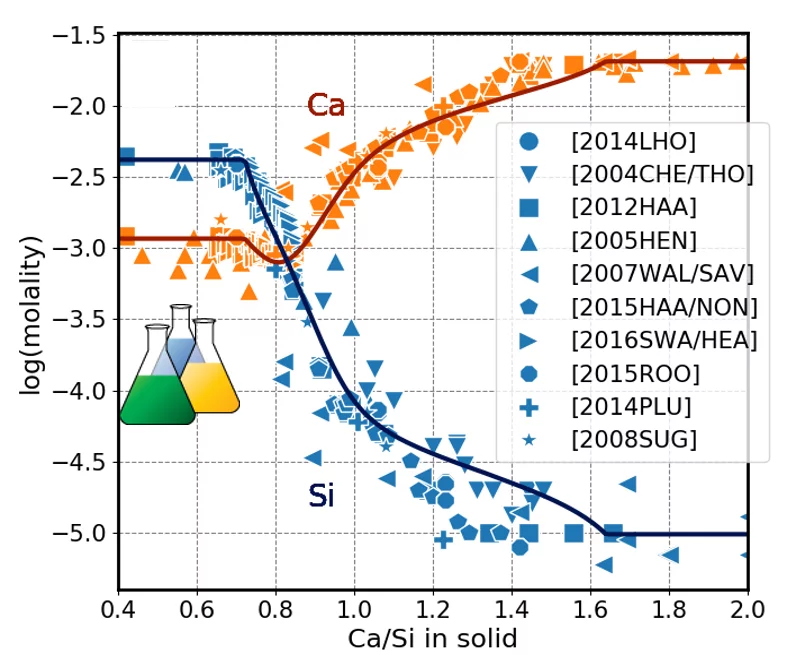
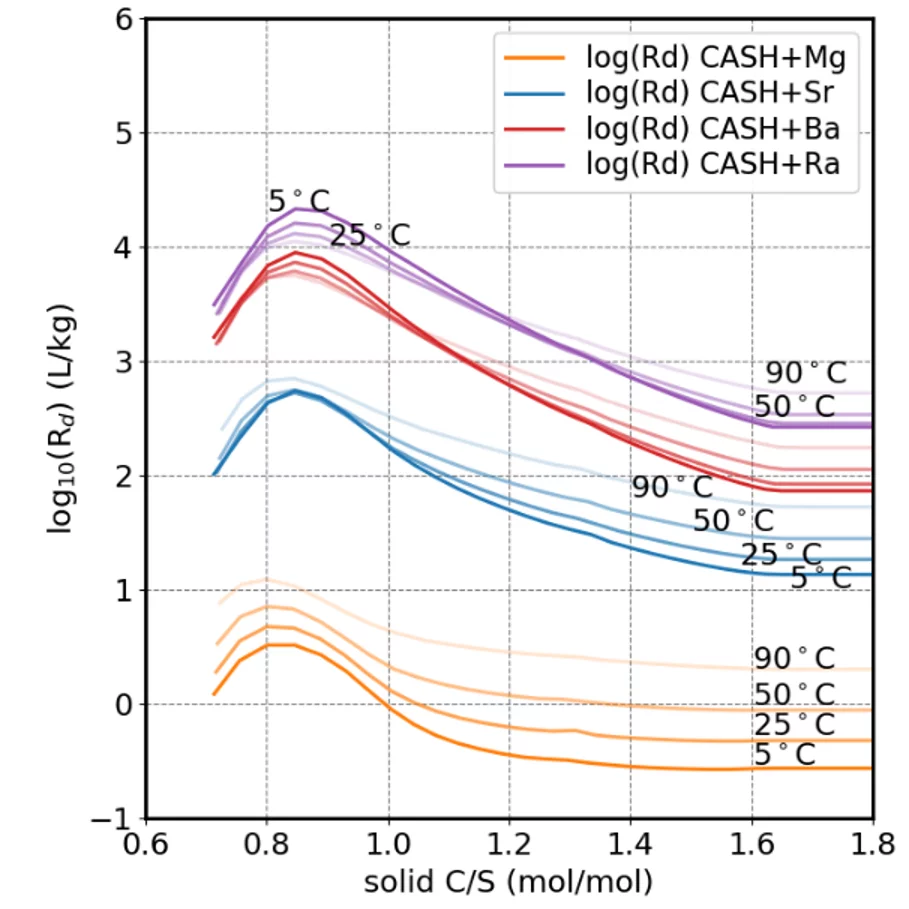
The CASH+ model can predict the uptake of different cations (Fig.2) and can be use to model the evolution of cement upon hydration (Fig.3).
Currently, the model includes Ca, Si, H2O, Al, Fe, Na, K, Li, Cs, Mg, Ba, Ra, Sr; work is on-going on adding actinides U, Np; as well as Zn and other hazardous metals. This is currently the most advanced and accurate chemical thermodynamic model for C-A-S-H worldwide.
The model will be added to the basis for the safety assessment studies in the final stage of the general license application is Switzerland.
Author
Mr. George Dan Miron
+41 56 310 24 32
dan.miron@psi.ch
Laboratory Waste Management, LES
Geosphere Transport
Original Publication
Kulik, D. A., Miron, G. D., & Lothenbach, B. (2022). A structurally-consistent CASH+ sublattice solid solution model for fully hydrated C-S-H phases: Thermodynamic basis, methods, and Ca-Si-H2O core sub-model. Cement and Concrete Research, 151, 106585. https://doi.org/10.1016/j.cemconres.2021.106585
Miron, G. D., Kulik, D. A., Yan, Y., Tits, J., & Lothenbach, B. (2022). Extensions of CASH+ thermodynamic solid solution model for the uptake of alkali metals and alkaline earth metals in C-S-H. Cement and Concrete Research, 152, 106667. https://doi.org/10.1016/J.CEMCONRES.2021.106667
Miron, G. D., Kulik, D. A., & Lothenbach, B. (2022). Porewater compositions of Portland cement with and without silica fume calculated using the fine-tuned CASH+NK solid solution model. Materials and Structures 2022 55:8, 55(8), 1–13. https://doi.org/10.1617/S11527-022-02045-0