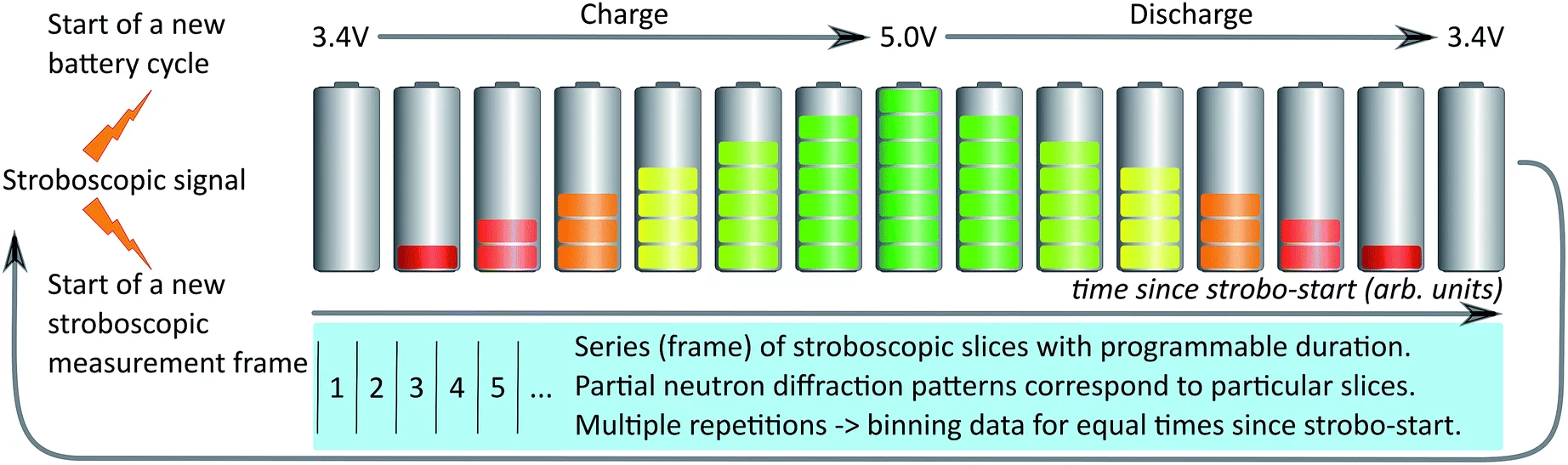
The first application of stroboscopic neutron diffraction to studying lithium-ion batteries during operation establishes a new approach to unravelling the complex processes playing out in energy-storage materials.
It is one of those things that never can go fast enough: charging your phone, computer or car. What limits the speed at which a battery can be charged, or discharged, depends on a complex interplay of electrochemical processes in the cell, some of which change substantially as the materials involved degrade. Neutron diffraction has long been recognised as a powerful modality for following structural modifications of individual components as batteries are going through charge–discharge cycles. One problem though has been that especially during fast cycling, there is simply not enough time to acquire sufficient neutron-diffraction data for extracting structural information about intermediate states. Writing in the Journal of Materials Chemistry A [1], Denis Sheptyakov and Vladimir Pomjakushin from the Laboratory for Neutron Scattering and Imaging (LNS), working with colleagues from the Electrochemistry Laboratory (LEC) at PSI and an industrial partner, present a promising route to easing that problem. They show that by applying a stroboscopic technique — whereby the same stage of a periodic process is sampled over several iterations — they can obtain appropriate statistics to characterise short-lived states during fast charging and discharging.
Understanding though repetition
Neutrons are a natural probe for battery components, as they can easily penetrate bulk materials and provide detailed quantitative information about their crystal structure. In earlier collaborative works, LEC and LNS groups have designed cells for studying battery materials during operation in cell geometries that are comparable to those of commercial systems. However, the sort of processes that could be followed within these cells was limited by the relatively low signal intensity of neutron diffraction experiments. Neutron diffraction datasets of sufficient quality for performing structure refinement typically take at least several minutes to acquire, but the time needed might stretch to hours. This is a problem when studying battery cells during charging and discharging, where some of the intermediate states of the materials of interest exist only for less than a minute.
In order to address that problem, Sheptyakov, Pomjakushin and co-workers have implemented at the High-Resolution Powder Diffractometer for Thermal Neutrons (HRPT) of SINQ techniques that make it possible to operate the detector in a stroboscopic mode. That is, the acquired data is time-stamped relative to a ‘start’ signal. If now the system under investigation can be repeatedly sent through identical cycles, with a new trigger for each iteration, then data from corresponding time frames can be combined to yield a dataset with sufficient intensity for subsequent structural (Rietveld) refinement. In the current realization of the HRPT data-handling system, time frames can be as short as ~10 ms, and developments are underway for re-binning the data after acquisition, such that they can be referenced relative to parameters other than just the time elapsed since the start signal.
Hard-charging regime
For batteries, a natural loop for stroboscopic sampling is one complete charge–discharge cycle (see the figure). In the lithium-ion batteries studied by the PSI team, data were typically collected with binnings on the order of tens of seconds. While ‘single-shot’ diffraction patterns were not of a quality suitable for Rietveld refinement, merging data from several consecutive charge–discharge cycles produced sets with sufficient statistics to determine the crystal structure of both the anode and cathode materials (graphite and disordered LiNi0.5Mn1.5O4, respectively).
Looking at different time frames within a cycle, the researchers followed the structural evolution of the materials at a microscopic level. Importantly, they were able to do so for unusually fast rates, where the time for fully charging or fully discharging was only around 12 minutes. When they pushed even harder, to a regime of one discharge within 4 minutes, they still could extract some qualitative information, but the limits of their approach were reached. This not least because fast cycling also means fast aging of the materials. In how far the electrochemical mechanisms at these extreme rates differ from those observed at more moderate ones promises to provide unique clues about how quickly lithium-ion batteries can be charged and discharged in practice, without unduly sacrificing battery life.
This work was carried out in close collaboration with Claire Villevieille from the Université Grenoble Alpes and the industrial partner Saft.
Contact
Dr. Denis Cheptiakov
Paul Scherrer Institut, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 3070, e-mail: denis.cheptiakov@psi.ch
Original Publication
1. Stroboscopic neutron diffraction applied to fast time-resolved operando studies on Li-ion batteries (d-LiNi0.5Mn1.5O4vs. graphite)
D. Sheptyakov, L. Boulet-Roblin, V. Pomjakushin, P. Borel, C. Tessier, C. Villevieille
J. Mater. Chem. A 8, 1288 (2020).
DOI: 10.1039/c9ta11826h