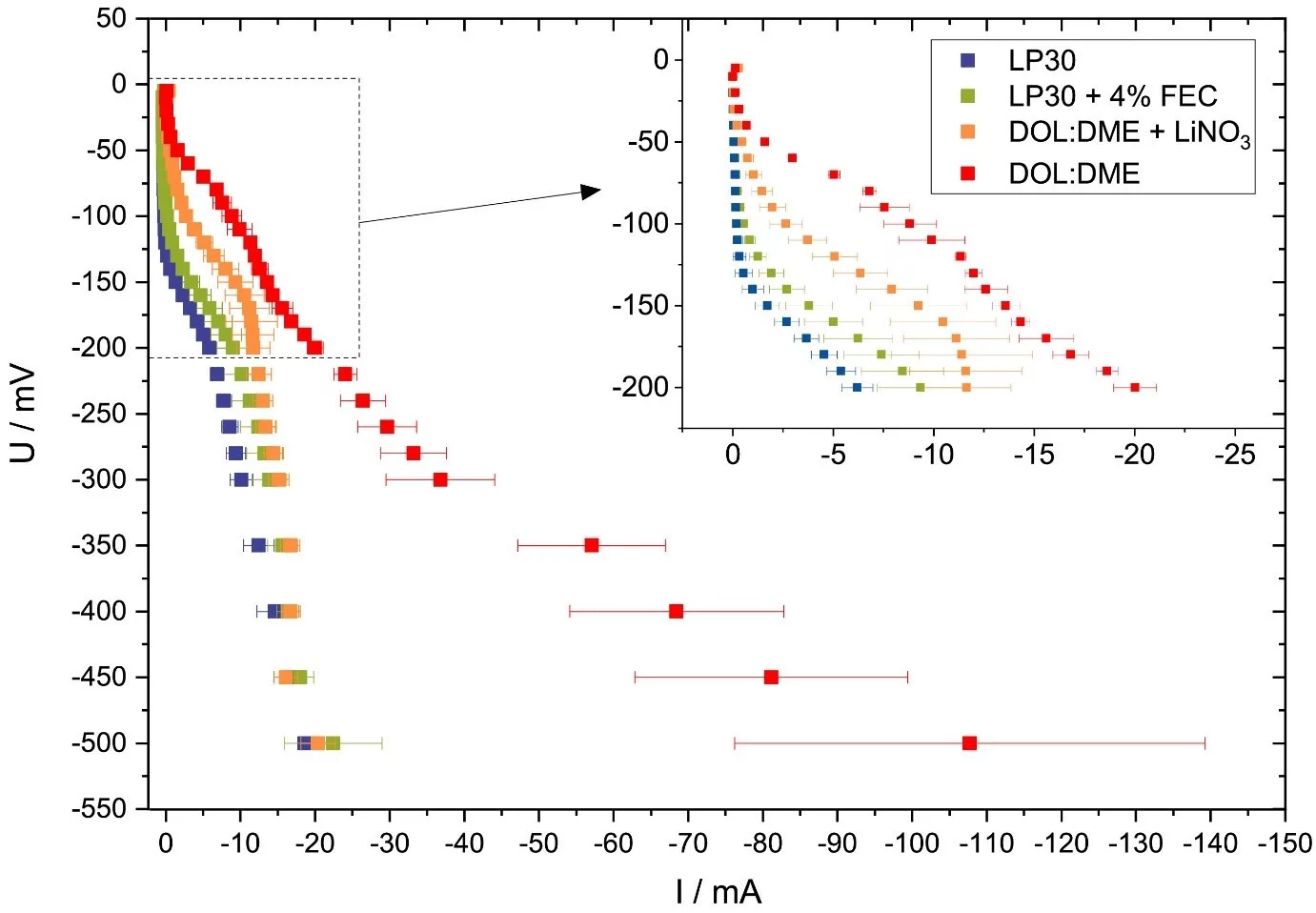
Evaluating potential electrolyte candidates is typically a lengthy procedure requiring long-term cycling experiments. To speed this process up, we have investigated potentiostatic lithium plating as a potential method for fast electrolyte suitability investigation. The applications of this methodology is not limited to liquid electrolytes, - effects of solid-state electrolytes, coatings, and other modifications can be readily assessed.
In many applications, lithium-ion batteries are currently a preferred choice due to their high energy density, high round-trip efficiency and reasonable cycle lifetimes. The next step to higher energy density will not be achieved by optimizing conventional LIB designs based on graphite negative and transition metal positive electrodes, and therefore new solution are needed. One of the “new” materials, able to significantly increase energy density of the battery is metallic lithium, considered as the ‘holy grails’ in the battery field due to its highly negative potential (−3.04 V vs. SHE) as well as high specific capacity (3860 mAh g−1), both of which can potentially double the energy density of already-existing Li-ion batteries.
There are numerous approaches to improve metallic lithium reversibility, where modification of the electrolyte composition is one of most studied: without an adequate additive, existing carbonate-based chemistries rather accelerate degradation processes and dendritic deposition than prevent it. On the other hand, ether-based systems allow for higher cycling stability, but their limited electrochemical stability window hinders using standard cathode materials as positive electrodes and because of that prevents achieving high energy density. Therefore, finding a fully compatible electrolyte for LMBs is considered one of the main challenges.
Nonetheless, the development of lithium-metal-compatible electrolytes is not a trivial process. A complete evaluation of the suitability of a single electrolyte typically requires multiple methods and methodologies, such as electrochemical stability tests, long-term cycling, impedance measurements, surface analysis, and computational studies. This can make screening of electrolytes, while varying their composition, a complex and time-consuming process, essentially forming a bottleneck for advancements in the field. This gave us the idea to start looking at potential methodologies that would allow us to screen an electrolyte and obtain information about its impact on the kinetics of lithium plating within a matter of hours.
In an unusual approach, we let ourselves inspire from the so-called ‘electrochemical hydrogen pumping’ experiment, which is commonly applied in the field of fuel cells. While in the vast majority of experiments on lithium metal anodes, standard galvanostatic plating is being employed; in electrochemical hydrogen pumping, the driving force of reactions is the potential. While the idea of potentiostatic pulse plating is not completely new, we are taking a more holistic look by not just looking at ultrafast kinetics, but instead seek to characterise different electrolytes. This we have achieved by applying a fixed, incrementally increasing potential to coin-type cells, while letting the current flow freely during this process. The resulting potential-versus-current curve, which closely resembles a typical cell polarisation curve, helps us to obtain information about the lithium deposition kinetics in the electrolytes under evaluation.
In a short experiment, valuable information on the kinetics of Li deposition, such as activation barrier and overpotential, as well as exchange currents, could be obtained. Repeated cycling also showed that there is a close correlation between stepwise deposition of fresh Li layers on cycled electrodes with inactive, so called ‘dead’, lithium, and the measured current response curve. In already cycled electrodes, additional activation barriers - as compared to pristine current collector - were observed, each corresponding to the nucleation of a new, island-like metal layer on top of ‘dead’ Li. In addition, when looking at a benchmark of several ether and carbonate electrolytes, electrolyte-specific current responses were observed. In addition to tracking the progress of Li deposition, potentiostatic plating is a helpful tool for comparative experiments, especially due to the close relation between the onset potential of nucleation and the self-diffusion coefficient of Li. Hereby, high self-diffusion corresponded to early deposition of the metal. Lastly, it was also found that addition of 4 wt.% fluoroethylene carbonate additive to commercial carbonate electrolyte brings its kinetic properties much closer to the well-behaved ether electrolytes, which are generally considered a better choice for metallic Li cycling. The applications of this methodology is not limited to liquid electrolytes, - effects of solid-state electrolytes, coatings, and other modifications can be readily assessed.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publications
Potentiostatic Lithium Plating as a Fast Method for Electrolyte Evaluation in Lithium Metal Batteries
Eric Winter, Thomas J. Schmidt, Sigita Trabesinger
Electrochim. Acta, in Press (2022) 141547.
DOI: 10.1016/j.electacta.2022.141547