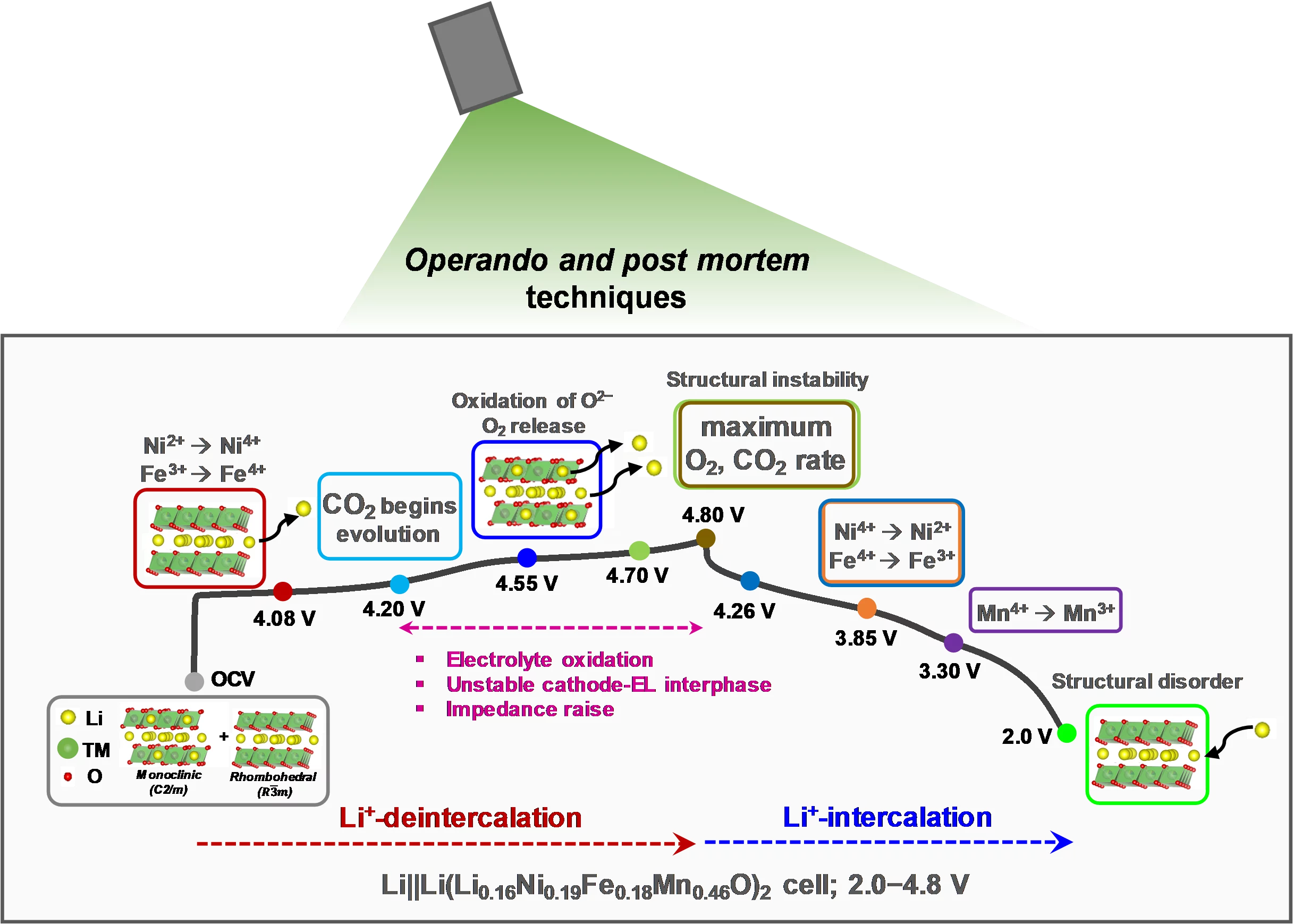
Lithium-rich layered oxides, containing cobalt, despite being promising high-capacity cathode materials, need alternatives to eliminate toxic and geopolitically restricted cobalt. An ongoing search for low-cost, Co-free Li-rich cathode materials with a better structural stability lead to investigation of Li1.16Ni0.19Fe0.18Mn0.46O2 (LNFM), where cobalt is replaced by abundant iron. Our LNFM not only delivered a high capacity of 229 mAh/g but also has a stable average discharge voltage when cycled to upper cutoff potential of 4.8 V in additive-free electrolyte.
High-energy-density lithium-ion batteries are in demand due to ongoing mass electrification of transport sector. As battery cathode materials play a vital role boosting the energy density of Li-ion cells, Li-rich layered oxides xLi2MnO3∙(1−x)LiNi1−y−zCoyMnzO2 (LR-NCMs) have received a lot of attention due to their high specific capacity and high energy density (up to 250 mAh/g and 900 Wh/kg on material level), when charged to above 4.6 V vs. Li+/Li. During the initial charge, the LR-NCM cathodes show a plateau region at ≈4.5 V in their voltage profile, corresponding to an irreversible oxidation of the lattice oxygen. Such oxygen redox reactions result in a lower average oxidation state of the TMs at the end of the first discharge, facilitating a higher reversible capacity in the following cycles beyond the theoretical one.
However, although LR-NCM cathodes can deliver extra capacity via oxygen redox, at the same time, these reactions can induce irreversible capacity loss and oxygen evolution, causing structural degradation upon long-term cycling. In addition, the practical applications of the LR-NCM cathode materials are hindered by toxic nature of Co with its geopolitical restrictions and related high-cost. Several strategies have been developed to improve the electrochemical performance of LR-NCM, which are, for example, surface coating, doping by small fraction of atoms, various electrolyte additives, etc. However, these did not resolve capacity and voltage fade upon long-term cycling, especially with high cut-off potential. All this has motivated the search for alternative high-capacity Co-free cathode materials with lower cost, better environmental friendliness, and without severe capacity and voltage fade.
In this regard, Co-free Fe–Mn based Li-rich cathodes (LNFM) are considered promising materials in the aspects of performance and cost, mainly due to the abundance, cheapness and environmental friendliness of Fe. So far, various LNFM cathode materials have been prepared using different synthesis methods, and the effect of Fe for improvement of the electrochemical performance was primarily studied. Therefore, we concluded that there is a need to understand the mechanism of oxygen redox reactions and their effects on the interfacial structure of LNFM, as well as establish how these correlate to the cycling performance of in Co-free cathodes. Therefore, in this study, we have synthesized Co-free Fe-Mn–based Li-rich cathode material Li1.16Ni0.19Fe0.18Mn0.46O2 (LNFM) by a simple co-precipitation method.
The identification of initial structural changes, gas evolution and oxygen redox, as well as cathode−electrolyte interfacial reactions and their correlation to improved electrochemical performance led to mechanistic understanding of LNFM cathode material. By using operando/ex situ and in situ both XRD and Raman techniques, it is concluded that, upon Li+-de(intercalation), LNFM layered structure remains dominant and there is no significant spinel formation during the initial cycles, as well as upon long-term cycling. The combination of operando OEMS and in situ EIS experiments allowed us to identify both CO2, O2 and POF3 gases and corresponding interfacial reactions at the cathode−electrolyte interface during the initial cycling of LNFM cathode. These results show that the weaker oxygen evolution lowers the conversion rate of LNFM layered structure to the spinel-like phase, alleviating voltage and capacity fade during cycling. Such structural stability along with sufficient interfacial stability lead to the improved electrochemical performance in half-cells. To further highlight the advantages offered by the LNFM cathode material, a full-cell comprising high-capacity LNFM cathode and graphite anode was electrochemically tested. Our LNFM not only delivered a high capacity of 229 mAh/g but also has a stable average discharge voltage when cycled to upper cutoff potential of 4.8 V in additive-free electrolyte. We believe that this work provides new insights into the available high performance Co-free LNFM cathode materials with simple synthesis procedure and already excellent performance even without electrolyte optimization.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publications
Correlating the Initial Gas Evolution and Structural Changes to Cycling Performance of Co-free Li-rich Layered Oxide Cathode
Hieu Quang Pham, Łukasz Kondracki, Mohamed Tarik, Sigita Trabesinger
Journal of Power Sources, 527 (2022) 231181.
DOI: 10.1016/j.jpowsour.2022.231181
Acknowledgement
This work was financially supported by the The Swiss Competence Center for Heat and Electricity Storage (SCCER-HaE).