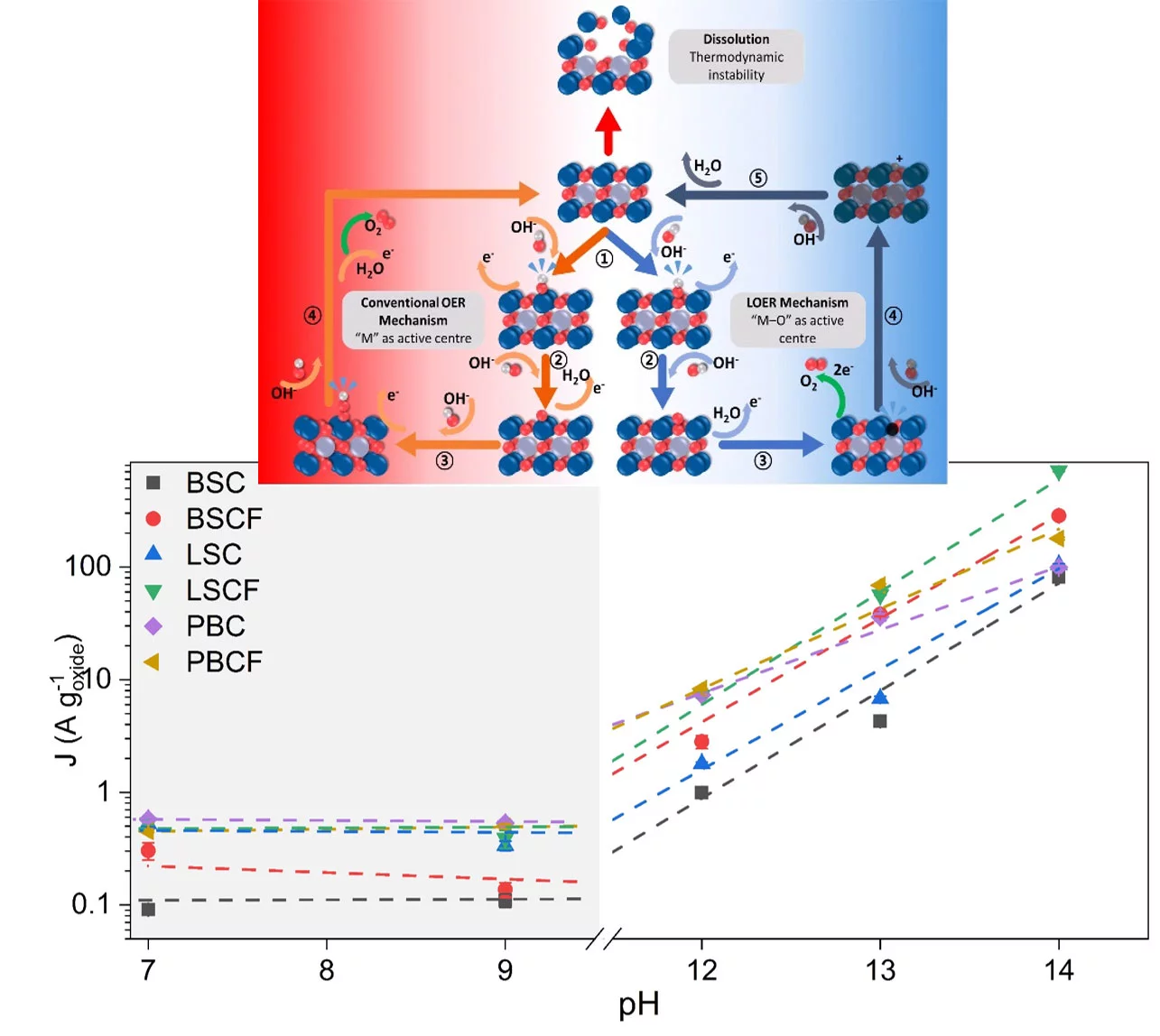
PSI researchers have studied the how the electrolyte pH values influence the oxygen evolution reaction (OER) activity and stability of different promising perovskite oxide catalysts for application as anodic electrodes in alkaline water electrolyzers. The OER activity and stability decreased decreasing the electrolyte pH values. By combining electrochemical studies and operando X-ray absorption spectroscopy measurements, it has been suggested that different reaction mechanisms dominate in alkaline and near-neutral electrolyte pH region.
Energy conversion and storage technologies are drawing growing attention for mediating inconsistent power generation from renewable energy sources. Water electrolysis utilizes hydrogen as an energy vector to store large amounts of energy, and typically operates either in acidic or alkaline environment. Particularly, operation under alkaline conditions allows exploring abundant and low cost non-noble metals as electrocatalysts, such as perovskite oxides. However, KOH solutions as electrolyte presents vulnerability to carbonation during operation, which shifts the pH level towards near-neutral values and decreases the electrolyte conductivity. In the present study the oxygen evolution reaction (OER) activity and stability versus different electrolyte pH levels have been investigated for a series of perovskite oxides, namely Ba0.5Sr0.5CoO3-δ (BSC), Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF), La0.5Sr0.5CoO3-δ (LSC), La0.2Sr0.8Co0.8Fe0.2O3-δ (LSCF), PrBaCo2O6-δ (PBC), and PrBaCo1.6Fe0.4O6-δ (PBCF) prepared via flame spray synthesis. The studied perovskites showed a first-order relationship between OER activity and electrolyte pH in the high alkaline region while revealing zeroth-order relationship in the near-neutral pH region. Differences in the OER activity versus pH, suggest that different mechanisms are predominant in the different pH regions. From operando X-ray absorption spectroscopy (XAS) measurements of the catalysts during the OER process, we could observed the development of Co/Fe-oxy(hydroxide) species only alkaline environment. The formation of an oxyhydroxide layer on perovskite surface is generally associated to the occurrence of a different OER mechanism than the conventional one, which involves oxidation of lattice oxygen and it is named lattice oxygen evolution reaction (LOER) mechanism. Differently, in the near neutral region the development of a Co/Fe-oxy(hydroxide) surface layer was not visible. This suggests that the studies perovskite catalysts are more inclined to evolve oxygen through the LOER mechanism in a highly alkaline condition, while in near-neutral environment the convetional OER mechanism dominates. However, a catalyst would not perform oxygen evolution through only one of the two mechanisms discussed in here, but it would rather preferentially and proportionally take both paths (conventional and LOER) as they are thermodynamically entangled.
Contact
Dr. Emiliana Fabbri, Scientist
Prof. Dr. Thomas J. Schmidt, Group Leader and Department Head
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 27 95
E-mail: emiliana.fabbri@psi.ch; thomasjustus.schmidt@psi.ch
Original Publication
Oxygen Evolution Reaction Activity and Underlying Mechanism of Perovskite Electrocatalysts at Different pH
Bae-Jung Kim, Emiliana Fabbri, Mario Borlaf, Daniel F. Abbott, Ivano E. Castelli, Maarten Nachtegaal, Thomas Graule, Thomas J. Schmidt
Mater. Adv.
DOI: 10.1039/D0MA00661K
Acknowledgement
The authors gratefully acknowledge the Swiss National Science Foundation through its Ambizione Program and the NCCR Marvel, the Swiss Competence Center for Energy Research (SCCER) Heat & Electricity Storage through Innosuisse, Switzerland, and Paul Scherrer Institute for financial contributions to this work, respectively. The authors thank the Swiss Light Source for providing beamtime at the SuperXAS beamline.