To improve the performance of single atom catalysts (SACs), the structure of their active sites under operative conditions needs to be better understood. For this, we have performed in situ X-ray absorption spectroscopy measurements using a modulation excitation approach selectively sensitive to the species involved in the electrochemical reactions. This has allowed us to study the structural changes undergone by two types of SACs, and to tie the observed differences to their catalytic activities.
The commercialization of electrochemical energy conversion technologies requires inexpensive catalysts for the reactions taking place in their electrodes, like O2- or CO2-electroreduction. Single atom catalysts (SACs) based on abundant elements like iron show a promising performance for these reactions, but their activity, stability and selectivity need to be further improved in order to implement them in commercial devices. This calls for a better understanding of the ways in which the structure of these SACs’ active sites change during operation.
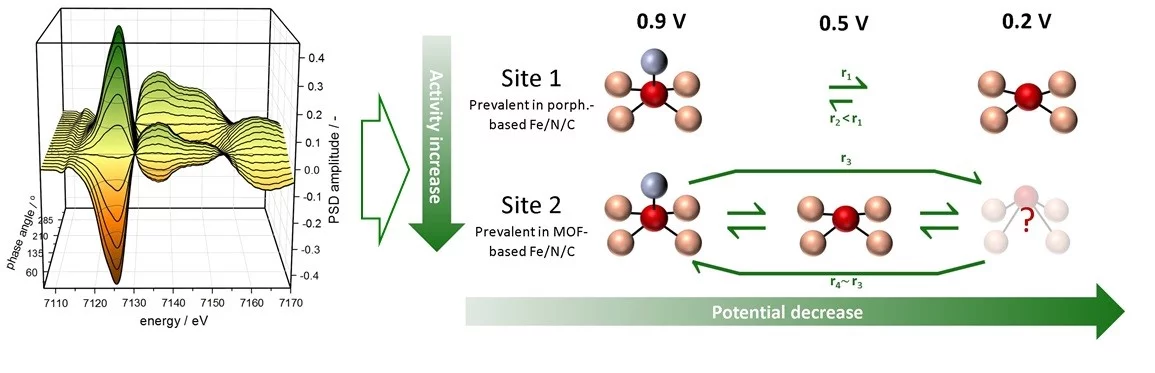
With this motivation, PSI researchers performed X-ray absorption spectroscopy (XAS) measurements at the Swiss Light Source to study how the electrochemical potential affects the structure of the sites in two types of SACs. Unlike in previous XAS studies, the PSI team applied a modulation excitation approach that yields results that are only sensitive to the sites that participate in the electrochemical reactions of interest. In doing so, they discovered that the sites in both SACs transform from a 5- to a 4-fold coordination when the potential is decreased from 0.9 to 0.5 V. In the case of the most active catalyst, though, further reducing the potential to 0.2 V leads to a displacement of the iron above the plane of its coordination environment that is not observed for the least active material. On top of this, the researchers found significant differences between the rates at which these structural changes take place in each catalyst. Specifically, the oxidation of the active sites is faster for the most active SAC, suggesting that the structural changes associated to this oxidation are tied to the slowest step in the complex mechanisms of these reactions. As a result, this study has unveiled a new parameter determining the catalytic performance of this kind of catalysts.
Contact
Dr. Juan Herranz, Senior Scientist
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 55 62
E-mail: juan.herranz@psi.ch
Original Publication
Time-Resolved Potential-Induced Changes in Fe/N/C Catalysts Studied by In Situ Modulation Excitation X-Ray Absorption Spectroscopy
Kathrin Ebner, Adam H. Clark, Viktoriia A. Saveleva, Grigory Smolentsev, Jingfeng Chen, Lingmei Ni, Jingkun Li, Andrea Zitolo, Frédéric Jaouen, Ulrike I. Kramm, Thomas J. Schmidt, and Juan Herranz
Adv. Energy Mater. 12, 2103699 (2022).
DOI: 10.1002/aenm.202103699
Acknowledgement
Swiss National Science Foundation, Ambizione Energy Grant No. PZENP2_173632.