Silicon is a long-standing candidate for replacing graphite as the active material in negative electrodes for Li-ion batteries, due to its significantly higher specific capacity. However, Si suffers from rapid capacity loss, as a result of the large volume expansion and contraction during lithation and de-lithiation. As an alternative to pure Si electrodes, Si could be used as a capacity-enhancing additive to graphite electrodes.
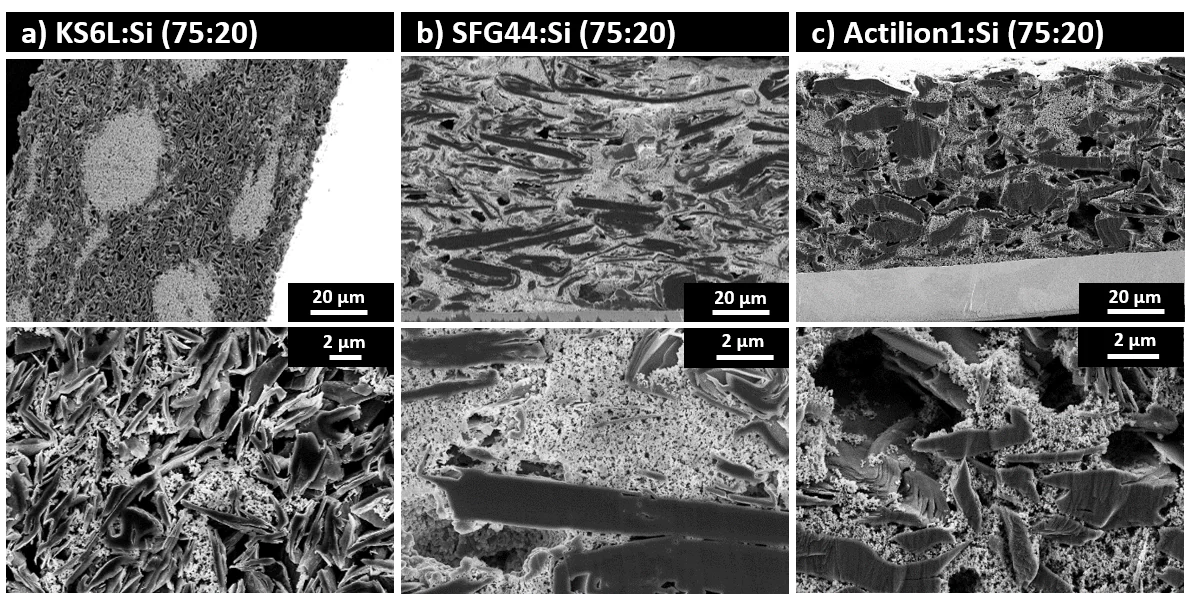
Such graphite–Si blend-electrodes have lower irreversible-charge losses during the formation of the solid electrolyte interphase (SEI) and maintain a better electronic contact within electrodes and with current collector. In this context we studied how the choice of graphite matrix can alter the cell performance. Varying the type of graphite and the Si content (5 or 20 wt%) results in different electrode morphologies and performance upon long-term cycling. In electrode blends comprising graphite and Si, graphite takes on the role as both active material and conductive additive. We have looked into several graphite properties, such as particle-size, particle-size distribution, conductivity, crystallinity and surface area. Our results demonstrate that, despite unfavorable electrode morphologies, such as large void spaces and poor active-material distribution, certain types of graphites with large particle sizes were found to be competitive with graphite–Si blends, containing smaller graphite particles. A broader particle-size-distribution within a specific graphite helps to achieve equally good capacity retention with an overall larger graphite particle-size, due to the ability of the fine fraction of the graphite to penetrate into the agglomerated Si domains, filling part of the void space created by the larger particles. In the presence of only 5 wt% Si, large Si domains are found between the large graphite particles, which are potential spots of increased stress and strain during the expansion of Si upon lithiation. A more homogeneous material intermixing was achieved only when graphites with smaller particle sizes were added to the electrodes. When the Si content is raised to 20 wt% and the graphite dimensions are in the range of tens of μm, the electrodes show an "inverse morphology," where the Si-fraction forms the electrode matrix with embedded graphite particles (graphite in silicon), see image. This is in stark contrast to electrodes containing smaller graphite particles, such as KS6L, where the main matrix is still provided by the graphite fraction (silicon in graphite), but which does not prevent Si agglomeration. Rate capability is always coming into the question when new materials are characterized; however, this question also arises when blends of different electroactive materials are used. As delithiated Si is considered a poor electronic conductor, one would assume that the presence of Si would induce additional internal resistance that hamper fast charging of this type of electrodes. We demonstrated, using current‐interruption measurements that the internal resistance of graphite‐Si blends indeed increases proportionally with the amount of Si in the electrode. And indeed, the additional resistance induced by the Si fraction in the active material blend impedes fast charging. Two strategies have been tested to mitigate the poor charging behavior at high rates: 1) the densification of the electrode coating and 2) higher contents of conductive additive. However, our results have shown that additional cell resistances, resulting in poor charging capability, do not solely originate from the presence of Si, because even the Si‐free, solely graphite electrodes are also lacking behind at the technologically‐feasible rates, despite the small potential gaps measured during the current‐interruption experiments. It is therefore likely that besides limitations of the active materials and the electrode architecture, intrinsic limitations of our cell setup are approached at high rates. Nonetheless, the experiments allowed a comprehensive comparison of fast charging limitations in graphite‐Si electrodes and an evaluation of the two common approaches to mitigate poor charge capability.
Contact
Dr. Sigita Trabesinger
Group Head Battery Electrodes and Cells
Paul Scherrer Institut
5232 Villigen PSI
Switzerland
Telephone: +41 56 310 57 75
E-mail: sigita.trabesinger@psi.ch
Original Publications
Graphite Particle-Size Induced Morphological and Performance Changes of Graphite–Silicon Electrodes
Fabian Jeschull, Yuri Surace, Simone Zürcher, Giacomo Lari, Michael E. Spahr, Petr Novák, Sigita Trabesinger
J. Electrochem. Soc. 167, 100535 (2020)
DOI: 10.1149/1945-7111/ab9b9a
Fast‐Charge Limitations for Graphite Anodes with Si as Capacity‐Enhancing Additive
Fabian Jeschull, Sigita Trabesinger
Batteries & Supercaps
DOI: 10.1002/batt.202000177
Acknowledgement
The authors gratefully thank Innosuisse (Project 18254.2).