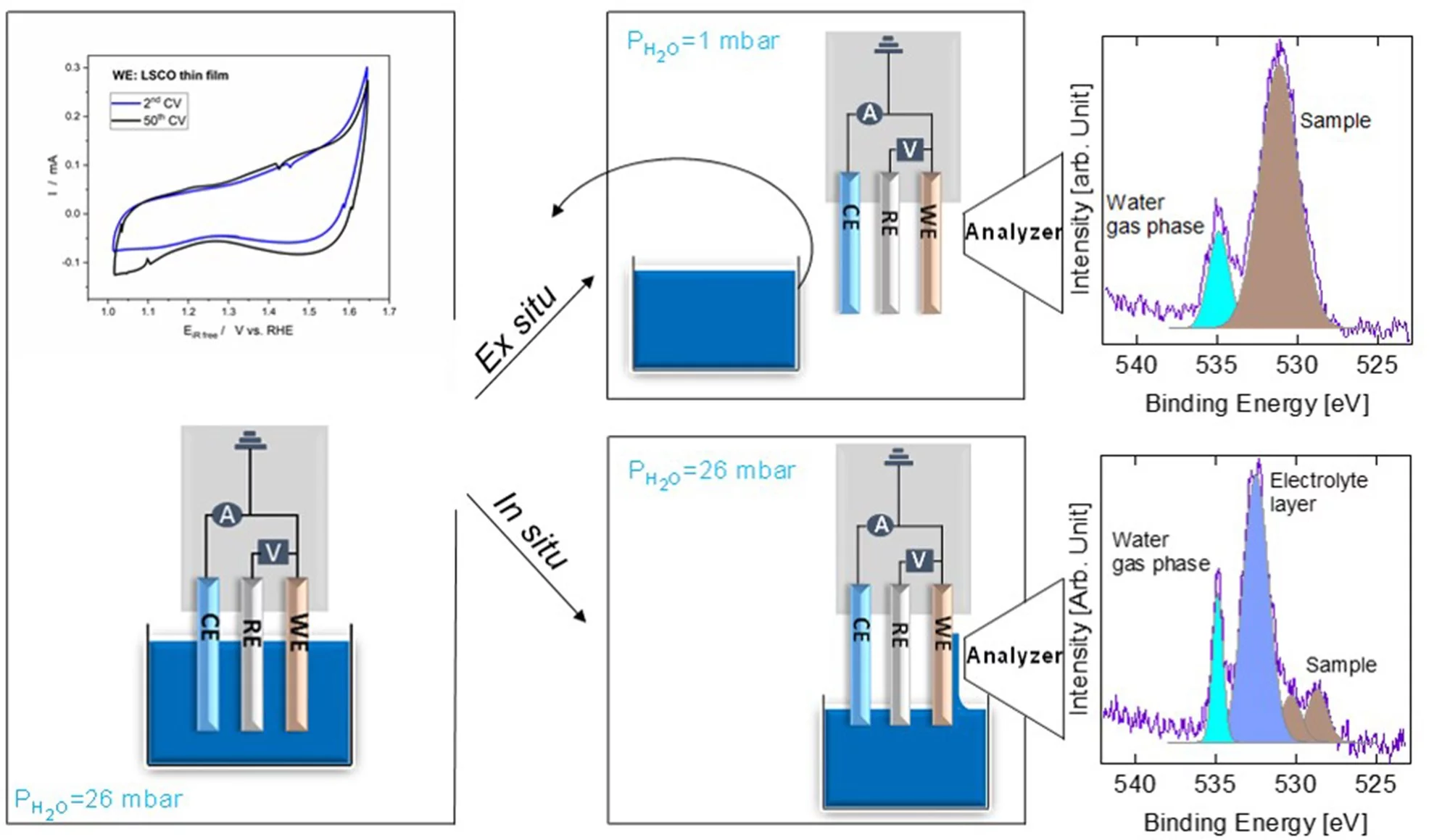
We carried out in situ and ex situ ambient pressure X-ray photoelectron spectroscopy (APXPS) experiments on a La0.2Sr0.8CoO3-δ perovskite oxygen evolution reaction (OER) catalyst. The study shows that Sr is leached into the electrolyte after immersion, leading to surface Co active site enrichment. Such a Co-enriched surface evolves into a new phase during operation. With the help of theoretical simulations, such a species is assigned to Co-oxyhydroxide, providing direct evidence of its formation during the OER.
Understanding the mechanism of the oxygen evolution reaction (OER) on perovskite materials is of great interest for the development of more active catalysts. The complexity of catalytic systems and scarce in situ and operando surface sensitive spectroscopic tools render the detection of active sites and the understanding of reaction mechanisms challenging. Recent progress in ambient pressure X-ray photoelectron spectroscopy (APXPS) allows the in situ surface investigation of OER catalysts. Particularly, by means of the “dip and pull” method, which makes use of a standard electrochemical three electrode setup, it is possible to simultaneously perform electrochemical and XPS characterizations. In the “dip and pull” method, a thin liquid electrolyte layer (10–30 nm thickness) is stabilized at the sample surface. This provides potential control of the working electrode while performing XPS measurements. However, in order to probe the buried interface of the electrode, it is necessary to generate photoelectrons with high kinetic energies, which can travel through the thin electrolyte layer and a vapor ambient pressure of 30 mbar. For this reason, tender X-rays (3000–10 000 eV) are typically used. Under OER conditions, OH ions in the thin electrolyte layer are quickly consumed and diffusion limitations from the bulk solution can lead to their depletion. This creates a high ohmic resistance that makes real operando measurements challenging. A second possibility for investigating catalyst surface changes induced by the OER is by performing XPS measurements ex situ directly after electrochemical measurements. In this case, XPS is not performed while a potential is applied to the working electrode. This ex situ approach overcomes drawbacks associated with the thin electrolyte meniscus, required for “dip and pull” measurements. Soft X-rays (< 2000 eV) can be utilized and their energy can be tuned to be sensitive to the topmost layers of the electrode, where active species form. In the present study, we have compared the results obtained by in situ and ex situ AP-XPS measurements on a La0.2Sr0.8CoO3- (LSCO) thin film perovskite electrode. LSCO is known to be a highly OER-active catalyst. The different experimental approaches provide complementary information that allow a complete characterization of the surface during and after the OER. Immersion of LSCO in the electrolyte solution leads to the leaching of surface segregated strontium species, leaving cobalt active sites exposed. Thanks to in situ and ex situ photoemission of O 1s performed during and after the OER, respectively, a new phase has been detected and assigned to surface cobalt oxyhydroxide. Such results have been confirmed by means of theoretical calculations of core electron binding energies. The direct observation of perovskite surface reconstruction during the OER, and the clear detection of a spectroscopic signature, undoubtedly indicate that the real active site for the perovskite OER activity is a Co-based oxyhydroxide layer. However, the perovskite structure is still visible underneath Co oxyhydroxide, suggesting an equilibrium between the two under the present experimental conditions. This study represents a clear example of in situ and ex situ surface sensitive measurements combined to detect active species formed during a reaction.
Contact
Dr. Emiliana Fabbri, Scientist
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 27 95
E-mail: emiliana.fabbri@psi.ch
Original Publication
Direct evidence of cobalt oxyhydroxide formation on a La0.2Sr0.8CoO3 perovskite water splitting catalyst
Anthony Boucly, Luca Artiglia, Emiliana Fabbri, Dennis Palagin, Dino Aegerter, Daniele Pergolesi, Zbynek Novotny, Nicolo Comini, J. Trey Diulus, Thomas Huthwelker, Markus Ammann, Thomas J. Schmidt
J. Mater. Chem. A, 2022, Advance Article
DOI: 10.1039/d1ta04957g
Acknowledgement
The post-doc position of AB was funded by the Energy and Environment division of the Paul Scherrer Institute. ZN, NC and JTD acknowledge Swiss National Science Foundation for project grant 200020_172641. JTD additionally acknowledges the support from European Union's Horizon 2020 program (FPRESOMUS – MSCA 801459). This work was performed at the PHOENIX I (X07MB) beamline and In situ Spectroscopy (X07DB) beamline of the Swiss Light Source, Paul Scherrer Institut.