CO2 capture from dilute gas mixtures (e.g., combustion flue gases, air) is increasingly recognized as a critical technological pathway towards stemming catastrophic climate change. Conventional thermal-based processes for removing CO2 from flue gas (e.g., amine scrubbing) are energy intensive and significantly reduce power plant efficiency. Electrochemical separation approaches have the potential to reduce these power requirements considerably by using electrons to transport CO2 as (bi)-carbonate ions across an alkaline membrane.
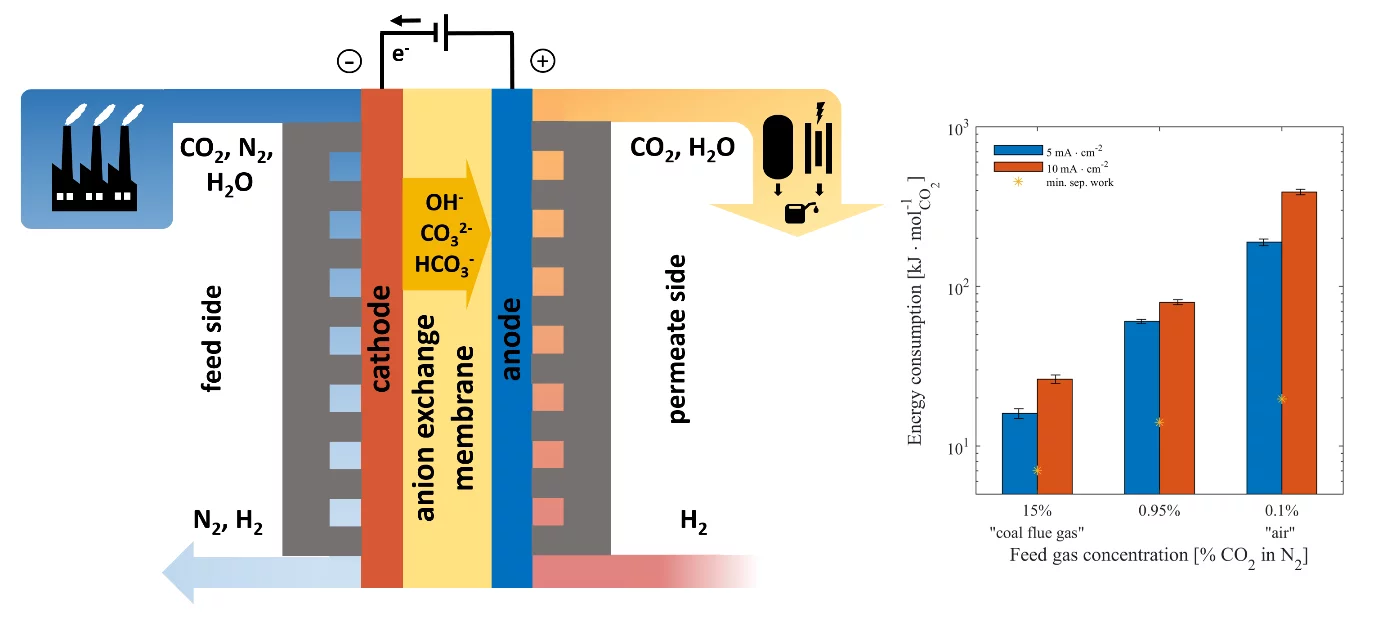
Electrochemically driven CO2 capture is a rapidly growing field of research that represents a wide yet largely unexplored parameter space. We have developed a first-of-its-kind methodology that uses an anion exchange membrane sandwiched between Pt/C catalyst-coated gas diffusion electrodes within a cell, as shown in Figure 1. OH- ions that are generated on the feed (cathode) side through the hydrogen evolution reaction (HER) react with CO2 molecules present in the feed gas stream to form HCO3− and CO32− ions (carbonation). These ions then electromigrate across the membrane to the permeate (anode) side, where they react with a stream of H2 to form a mixture of CO2 and H2O through the hydrogen oxidation reaction (HOR). The net result is the transport of CO2 from the feed side to the permeate side of the cell, where the resulting mixture of CO2 and residual H2 could be used for a downstream fuel synthesis process, such as methanol synthesis. We performed cell polarization experiments with current densities up to 50 mA·cm-2 using 0.1-100% CO2 in N2 as the feed gas, and permeate CO2 concentrations were monitored using on-line gas analysis. The bar plot in Figure 1 shows the molar specific energy consumption of the CO2 separation process (log scale) at the current densities of 5 and 10 mA·cm-2 for various feed gas concentrations, with the minimum theoretical separation work for the given gas mixture shown for reference. As shown in Figure 1, the energy consumption is heavily dependent on the feed gas concentration, with values of 16-26 kJ·mol-1 for 15% CO2 (similar to the concentration found in coal flue gas) and 189-390 kJ·mol-1 for 0.1% CO2 (similar to what is found in air). This is largely an effect of increased fraction of OH- pumping at low CO2 concentrations, which limits the applicability of the approach for sub-percent concentrations of CO2. Further experiments successfully demonstrated the feasibility of electrochemical CO2 pumping against concentration and pressure gradients. Finally, we employed a simplified techno-economic model to examine the cost dynamics of the system, showing that current densities of >50 mA·cm-2 and faradaic efficiencies of 50% are necessary for the process to be economically viable. The results represent an important step in mapping out the current capabilities and limitations of a membrane electrochemical process for CO2 capture, showing where efforts should be directed in future experimental campaigns.
Contact
Dr. Alexander Muroyama
Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villigen PSI
Telephone: +41 56 310 36 99
E-mail: alexander.muroyama@psi.ch
PD Dr. Lorenz Gubler
Head Membranes & Electrochemical Cells Group
Paul Scherrer Institut
5232 Villligen PSI
Telephone: +41 56 310 26 73
E-mail: lorenz.gubler@psi.ch
Original Publication
Separation of CO2 from Dilute Gas Streams Using a Membrane Electrochemical Cell
A.P. Muroyama, A. Beard, B. Pribyl-Kranewitter, L. Gubler
ACS ES&T Engineering 1(5), 905-916 (2021)
DOI: 10.1021/acsestengg.1c00048
Acknowledgement
The authors would like to acknowledge Shell Global Solutions BV for providing the funding for this work.