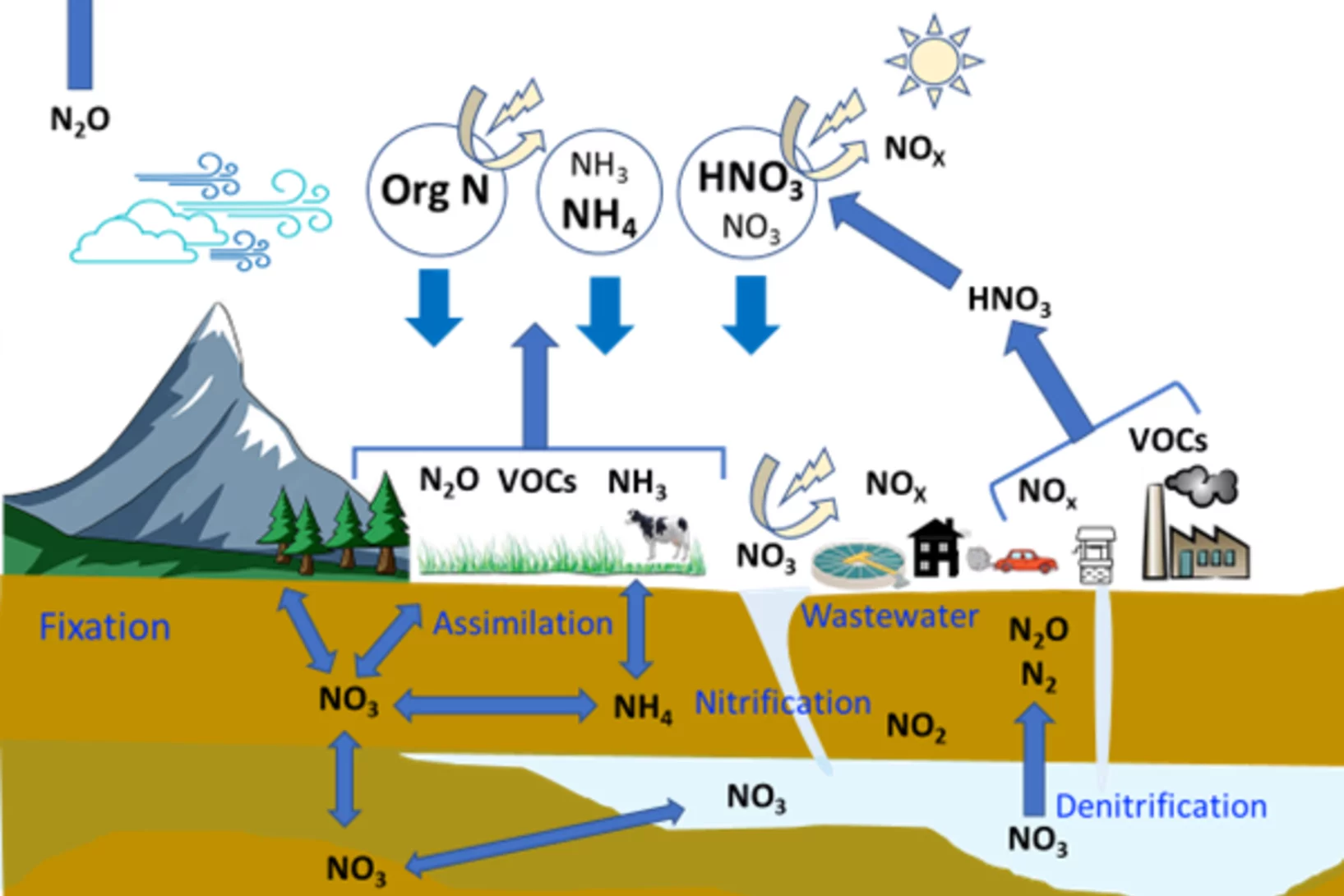
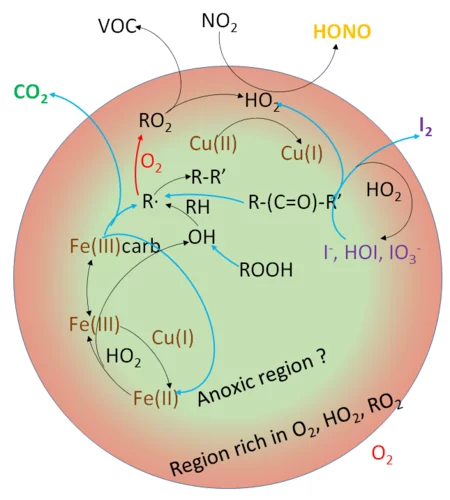
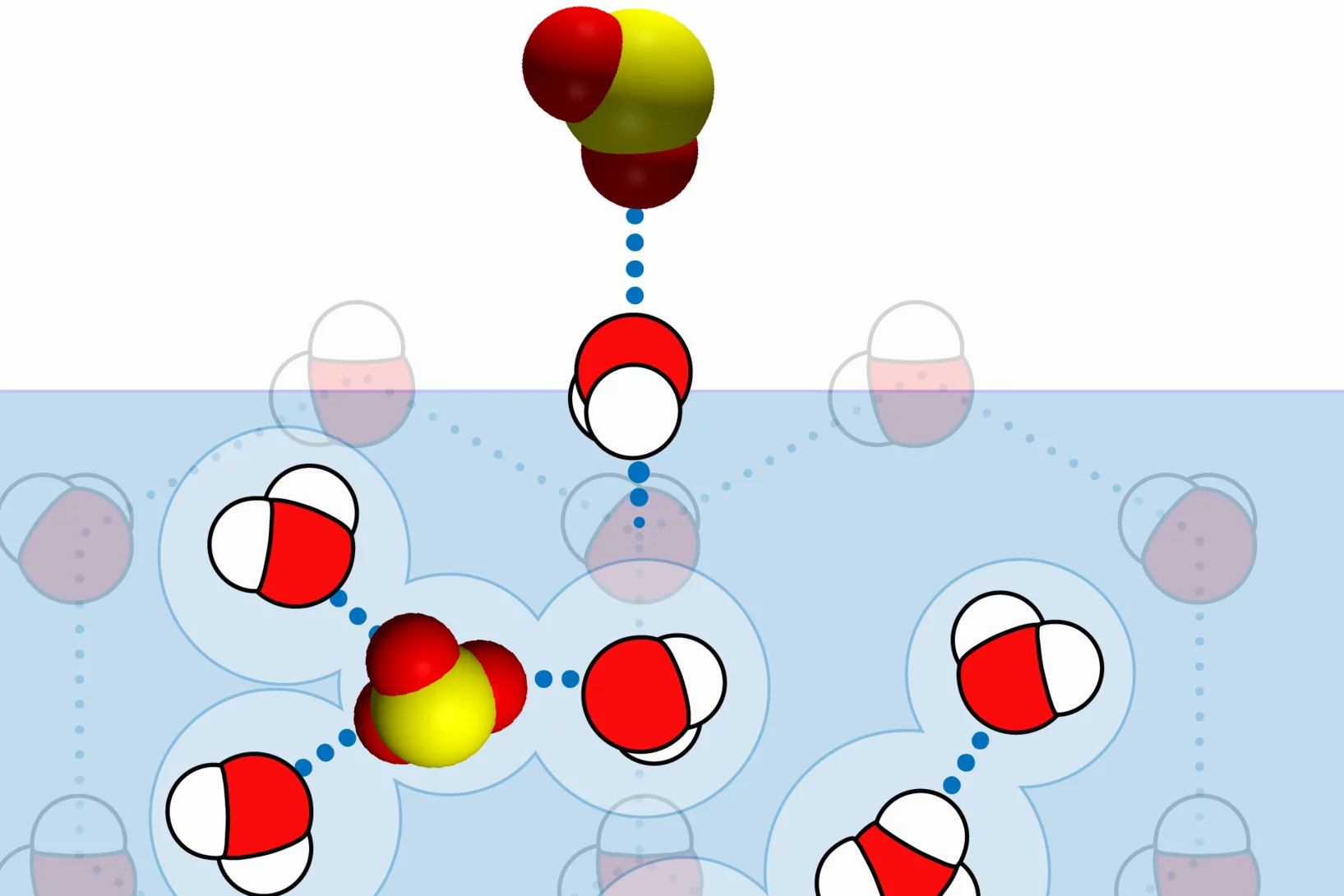
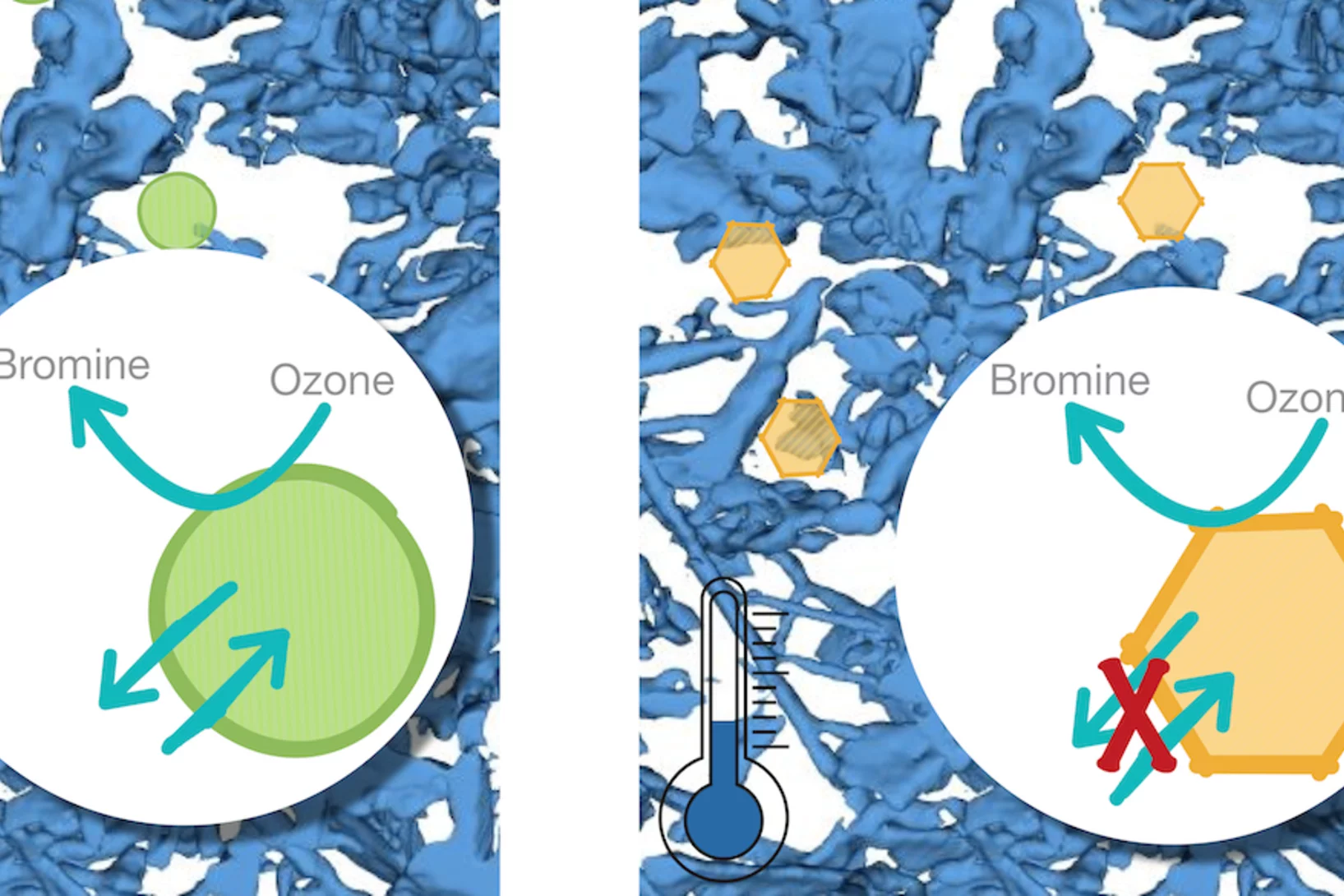
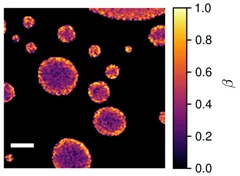
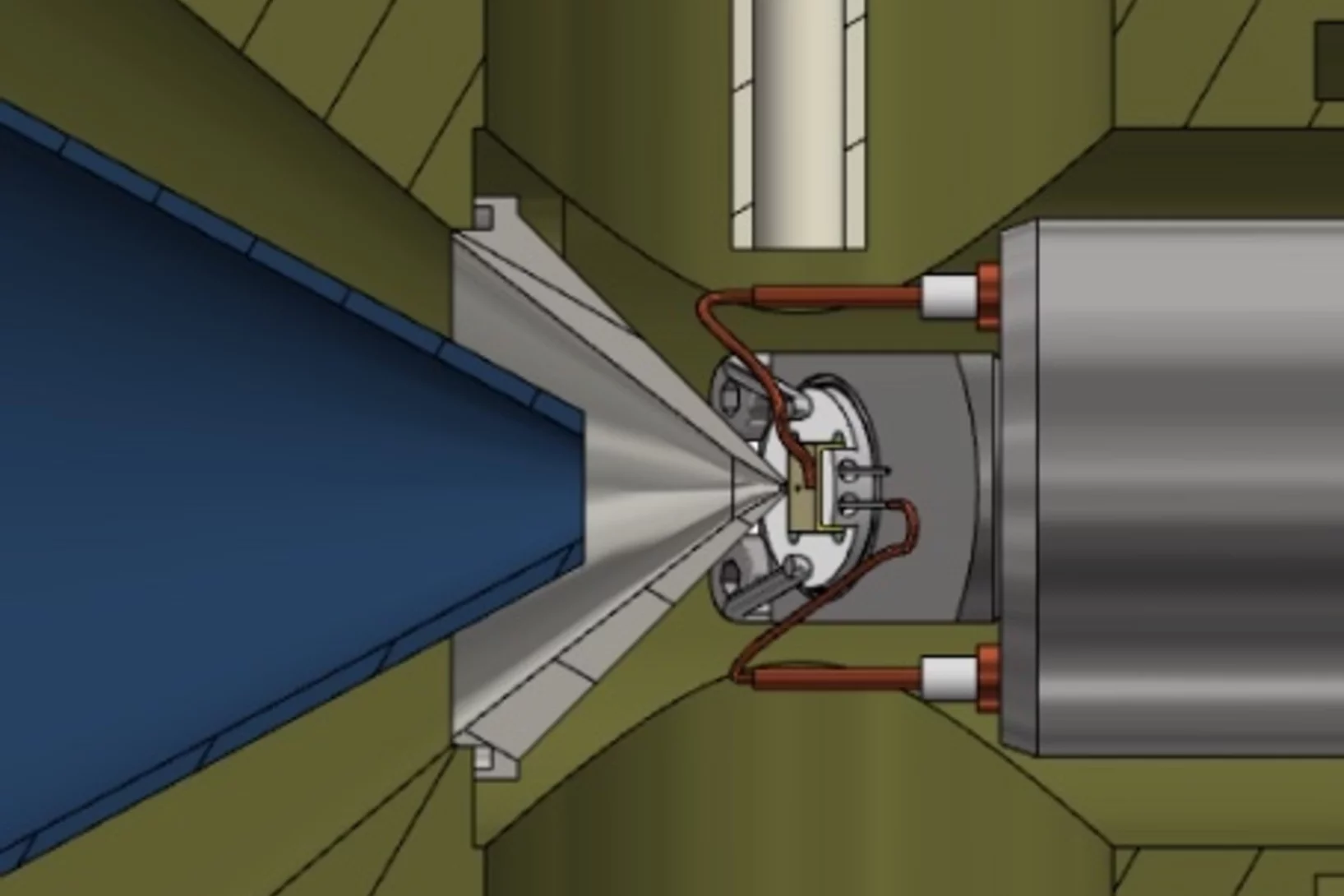
The Multiphase Chemistry group performs laboratory studies related to the chemistry, photochemistry and kinetics of atmospheric multiphase processes involving organic, halogen, nitrogen oxide and odd oxygen species on substrates relevant for aerosol particles, cirrus ice, ocean surface, soil surfaces, snow and sea ice. Understanding these processes is relevant for the assessment of the human impact on atmospheric composition, climate, human and ecosystem health.
We use a combination of in-situ spectroscopy, flow tube and chamber techniques to cover scales from molecular level world of interfaces to the microstructure in aerosol particles and to the mesosopic medium of snow.
We educate young scientists in laboratory atmospheric chemistry within the frame of the Institute of Atmospheric and Climate Sciences (IAC) at ETH Zürich (Department of Environmental Systems Sciences, D-USYS).
Projects
Team
Current Publications
A complete publication list is available at the institutional repository (DORA PSI).
-
Ammann M
Concluding remarks: atmospheric chemistry in cold environments
Faraday Discussions. 2025; 258: 597-613. https://doi.org/10.1039/d5fd00042d
DORA PSI -
Bartels-Rausch T, Creamean J, Thomas JL, Willis M, Zieger P
Ten crucial unknowns in atmospheric chemistry in the cold
Faraday Discussions. 2025; 258: 10-22. https://doi.org/10.1039/d5fd00056d
DORA PSI -
Chen S, Abouhaidar R, Artiglia L, Yang H, Boucly A, Iezzi L, et al.
Influence of surfactants with differently charged headgroups on the surface propensity of bromide
Journal of Physical Chemistry A. 2025; 129(13): 3085-3097. https://doi.org/10.1021/acs.jpca.4c07539
DORA PSI -
Garner NM, Mahrt F, Top J, Tadei V, Kilchhofer K, Takahama S, et al.
Photochemistry of iron-containing secondary organic aerosol is impacted by relative humidity during formation
npj Climate and Atmospheric Science. 2025; 8(1): 246 (9 pp.). https://doi.org/10.1038/s41612-025-01109-6
DORA PSI -
Kilchhofer K, Ammann M, Torrent L, Cheung RKY, Alpert PA
Copper accelerates photochemically induced radical chemistry of iron-containing secondary organic aerosol (SOA)
Atmospheric Chemistry and Physics. 2025; 25(14): 8061-8086. https://doi.org/10.5194/acp-25-8061-2025
DORA PSI -
Kilchhofer K, Barth A, Utinger B, Kalberer M, Ammann M
Reactive oxygen species buildup in photochemically aged iron- and copper-doped secondary organic aerosol proxy
Aerosol Research. 2025; 3(1): 337-349. https://doi.org/10.5194/ar-3-337-2025
DORA PSI -
Kong X, Ammann M, George C, Knopf D
Atomic and molecular ions in atmospheric chemistry: interfacial reactivity, emerging mechanisms, and future perspectives
Journal of Geophysical Research: Atmospheres. 2025; 130(20): e2025JD044379 (24 pp.). https://doi.org/10.1029/2025JD044379
DORA PSI -
Kuhn J, Stutz J, Bartels-Rausch T, Thomas JL, Cesler-Maloney M, Simpson WR, et al.
The interplay between snow and polluted air masses in cold urban environments
Faraday Discussions. 2025; 258: 502-520. https://doi.org/10.1039/d4fd00176a
DORA PSI -
Mahrt F, Nikkho S, Zaks J, Uppal G, Lam A, Ammann M, et al.
Surprising crystallinity of biomass burning secondary organic aerosol from catechol and nitrate radical reactions: evidence and possible implications
Environmental Science and Technology. 2025; 59(32): 16923-16932. https://doi.org/10.1021/acs.est.5c06834
DORA PSI -
Mishra A, Kilchhofer K, Iezzi L, Pöschl U, Alpert PA, Ammann M, et al.
Photochemical degradation of iron citrate in anoxic viscous films enhanced by redox cascades
ACS Earth and Space Chemistry. 2025; 9(3): 689-698. https://doi.org/10.1021/acsearthspacechem.4c00364
DORA PSI -
Pohl MO, Violaki K, Liu L, Gaggioli E, Glas I, von Kempis J, et al.
Comparative characterization of bronchial and nasal mucus reveals key determinants of influenza A virus inhibition
mSphere. 2025; 10(9): e0036525 (26 pp.). https://doi.org/10.1128/msphere.00365-25
DORA PSI -
Richter C, Gholami S, Manoharan Y, Buttersack T, Longetti L, Artiglia L, et al.
Uptake of ammonia by ice surfaces at atmospheric temperatures
Faraday Discussions. 2025; 258: 532-545. https://doi.org/10.1039/d4fd00169a
DORA PSI -
Vattioni S, Peter T, Weber R, Dykema JA, Luo B, Stenke A, et al.
Injecting solid particles into the stratosphere could mitigate global warming but currently entails great uncertainties
Communications Earth & Environment. 2025; 6(1): 132 (10 pp.). https://doi.org/10.1038/s43247-025-02038-1
DORA PSI -
Alpert PA, Corral Arroyo P, Ammann M
Microanalytical studies of multiphase reactions and particle aging
In: Conny JM, Buseck PR, eds. Microanalysis of Atmospheric Particles Microanalysis of atmospheric particles: techniques and applications. Geophysical monograph series. Hoboken: Wiley; 2024:201-222. https://doi.org/10.1002/9781119554318.ch10
DORA PSI -
Ammann M, Alpert PA, Artiglia L, Bao F, Bartels-Rausch T, Ospina JFF, et al.
Multiphase chemistry in the atmosphere
Chimia. 2024; 78(11): 754-761. https://doi.org/10.2533/chimia.2024.754
DORA PSI -
Buttersack T, Gladich I, Gholami S, Richter C, Dupuy R, Nicolas C, et al.
Direct observation of the complex S(IV) equilibria at the liquid-vapor interface
Nature Communications. 2024; 15(1): 8987 (8 pp.). https://doi.org/10.1038/s41467-024-53186-5
DORA PSI -
De Angelis D, Longetti L, Bonano G, Cresi JSP, Foglia L, Pancaldi M, et al.
A sub-100 nm thickness flat jet for extreme ultraviolet to soft X-ray absorption spectroscopy
Journal of Synchrotron Radiation. 2024; 31(Part 3): 605-612. https://doi.org/10.1107/S1600577524001875
DORA PSI -
Decker ZCJ, Alpert PA, Ammann M, Anet JG, Bauer M, Cui T, et al.
Emission and formation of aircraft engine oil ultrafine particles
ACS ES&T Air. 2024; 1(12): 1662-1672. https://doi.org/10.1021/acsestair.4c00184
DORA PSI -
Fauré N, Chen J, Artiglia L, Ammann M, Bartels-Rausch T, Kanji ZA, et al.
Formation of sodium chloride on the surface of sulfate-rich gobi desert salt in response to water adsorption
ACS ES&T Air. 2024; 1(11): 1373-1382. https://doi.org/10.1021/acsestair.4c00092
DORA PSI -
Garner NM, Top J, Mahrt F, El Haddad I, Ammann M, Bell DM
Iron-containing seed particles enhance α-pinene secondary organic aerosol mass concentration and dimer formation
Environmental Science and Technology. 2024; 58(38): 16984-16993. https://doi.org/10.1021/acs.est.4c07626
DORA PSI -
Knopf DA, Ammann M, Berkemeier T, Pöschl U, Shiraiwa M
Desorption lifetimes and activation energies influencing gas-surface interactions and multiphase chemical kinetics
Atmospheric Chemistry and Physics. 2024; 24(6): 3445-3528. https://doi.org/10.5194/acp-24-3445-2024
DORA PSI -
Nikkho S, Bai B, Mahrt F, Zaks J, Peng L, Kiland KJ, et al.
Secondary organic aerosol from biomass burning phenolic compounds and nitrate radicals can be highly viscous over a wide relative humidity range
Environmental Science and Technology. 2024; 58(49): 21702-21715. https://doi.org/10.1021/acs.est.4c06235
DORA PSI -
Reza M, Iezzi L, Finkenzeller H, Roose A, Ammann M, Volkamer R
Iodine activation from iodate reduction in aqueous films via photocatalyzed and dark reactions
ACS Earth and Space Chemistry. 2024; 8(12): 2495-2508. https://doi.org/10.1021/acsearthspacechem.4c00224
DORA PSI -
Sellegri K, Simó R, Wang B, Alpert PA, Altieri K, Burrows S, et al.
Influence of open ocean biogeochemistry on aerosol and clouds: recent findings and perspectives
Elementa: Science of the Anthropocene. 2024; 12(1): 00058 (35 pp.). https://doi.org/10.1525/elementa.2023.00058
DORA PSI -
Testa B, Durdina L, Alpert PA, Mahrt F, Dreimol CH, Edebeli J, et al.
Soot aerosols from commercial aviation engines are poor ice-nucleating particles at cirrus cloud temperatures
Atmospheric Chemistry and Physics. 2024; 24(7): 4537-4567. https://doi.org/10.5194/acp-24-4537-2024
DORA PSI -
Alpert PA, Bernard F, Connolly P, Crabeck O, George C, Kaiser J, et al.
Application of simulation chambers to investigate interfacial processes
In: Doussin J-F, Fuchs H, Kiendler-Scharr A, Seakins P, Wenger J, eds. A practical guide to atmospheric simulation chambers. Cham: Springer Nature; 2023:293-330. https://doi.org/10.1007/978-3-031-22277-1_8
DORA PSI -
Armanious A, Wang H, Alpert PA, Medaglia C, Peydayesh M, Zwygart AC-A, et al.
Trapping virus-loaded aerosols using granular material composed of protein nanofibrils and iron oxyhydroxides nanoparticles
Frontiers in Soft Matter. 2023; 3: 1143958 (10 pp.). https://doi.org/10.3389/frsfm.2023.1143958
DORA PSI -
Bartels-Rausch T, Ammann M
It is time to introduce the next generation of chemists to FAIR and open science
Chimia. 2023; 77(10): 694-696. https://doi.org/10.2533/chimia.2023.694
DORA PSI -
Bartels-Rausch T, Gabathuler JP, Yang H, Manoharan Y, Artiglia L, Ammann M
Removing gas-phase features in near ambient pressure partial Auger-Meitner electron yield oxygen K-edge NEXAFS spectra
Journal of Electron Spectroscopy and Related Phenomena. 2023; 264: 147320 (7 pp.). https://doi.org/10.1016/j.elspec.2023.147320
DORA PSI -
Chen J, Jiang H, Chen X, Wang J, Huang D, Lian C, et al.
A novel mechanism for NO2-to-HONO conversion on soot: synergistic effect of elemental carbon and organic carbon
Environmental Science and Technology Letters. 2023; 10(10): 878-884. https://doi.org/10.1021/acs.estlett.3c00624
DORA PSI -
Fauré N, Chen J, Artiglia L, Ammann M, Bartels-Rausch T, Li J, et al.
Unexpected behavior of chloride and sulfate ions upon surface solvation of Martian salt analogue
ACS Earth and Space Chemistry. 2023; 7(2): 350-359. https://doi.org/10.1021/acsearthspacechem.2c00204
DORA PSI -
Hong AC, Ulrich T, Thomson ES, Trachsel J, Riche F, Murphy JG, et al.
Uptake of hydrogen peroxide from the gas phase to grain boundaries: a source in snow and ice
Environmental Science and Technology. 2023; 57(31): 11626-11633. https://doi.org/10.1021/acs.est.3c01457
DORA PSI -
Jeong D, McNamara SM, Chen Q, Mirrielees J, Edebeli J, Kulju KD, et al.
Quantifying the contributions of aerosol- and snow-produced ClNO2 through observations and 1D modeling
ACS Earth and Space Chemistry. 2023; 7(12): 2548-2561. https://doi.org/10.1021/acsearthspacechem.3c00237
DORA PSI -
Kiland KJ, Mahrt F, Peng L, Nikkho S, Zaks J, Crescenzo GV, et al.
Viscosity, glass formation, and mixing times within secondary organic aerosol from biomass burning phenolics
ACS Earth and Space Chemistry. 2023; 7(7): 1388-1400. https://doi.org/10.1021/acsearthspacechem.3c00039
DORA PSI -
Knopf DA, Alpert PA
Atmospheric ice nucleation
Nature Reviews Physics. 2023; 5: 203-217. https://doi.org/10.1038/s42254-023-00570-7
DORA PSI -
Kong X, Gladich I, Fauré N, Thomson ES, Chen J, Artiglia L, et al.
Adsorbed water promotes chemically active environments on the surface of sodium chloride
Journal of Physical Chemistry Letters. 2023; 14(26): 6151-6156. https://doi.org/10.1021/acs.jpclett.3c00980
DORA PSI -
Kärcher B, Marcolli C, Mahrt F
The role of mineral dust aerosol particles in aviation soot-cirrus interactions
Journal of Geophysical Research: Atmospheres. 2023; 128(3): e2022JD037881 (15 pp.). https://doi.org/10.1029/2022JD037881
DORA PSI -
Mahrt F, Rosch C, Gao K, Dreimol CH, Zawadowicz MA, Kanji ZA
Physicochemical properties of charcoal aerosols derived from biomass pyrolysis affect their ice-nucleating abilities at cirrus and mixed-phase cloud conditions
Atmospheric Chemistry and Physics. 2023; 23(2): 1285-1308. https://doi.org/10.5194/acp-23-1285-2023
DORA PSI -
Mallet MD, Humphries RS, Fiddes SL, Alexander SP, Altieri K, Angot H, et al.
Untangling the influence of Antarctic and Southern Ocean life on clouds
Elementa: Science of the Anthropocene. 2023; 11(1): 00130 (18 pp.). https://doi.org/10.1525/elementa.2022.00130
DORA PSI -
Vattioni S, Luo B, Feinberg A, Stenke A, Vockenhuber C, Weber R, et al.
Chemical impact of stratospheric alumina particle injection for solar radiation modification and related uncertainties
Geophysical Research Letters. 2023; 50(24): e2023GL105889 (10 pp.). https://doi.org/10.1029/2023GL105889
DORA PSI -
Yao Y, Alpert PA, Zuend A, Wang B
Does liquid-liquid phase separation impact ice nucleation in mixed polyethylene glycol and ammonium sulfate droplets?
Physical Chemistry Chemical Physics. 2023; 25(1): 80-95. https://doi.org/10.1039/d2cp04407b
DORA PSI