Alvra Research Group
News & Scientific Highlights
Tender X-rays show how one of nature’s strongest bonds breaks
Short flashes of an unusual kind of X-ray light at SwissFEL and SLS bring scientists closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical.
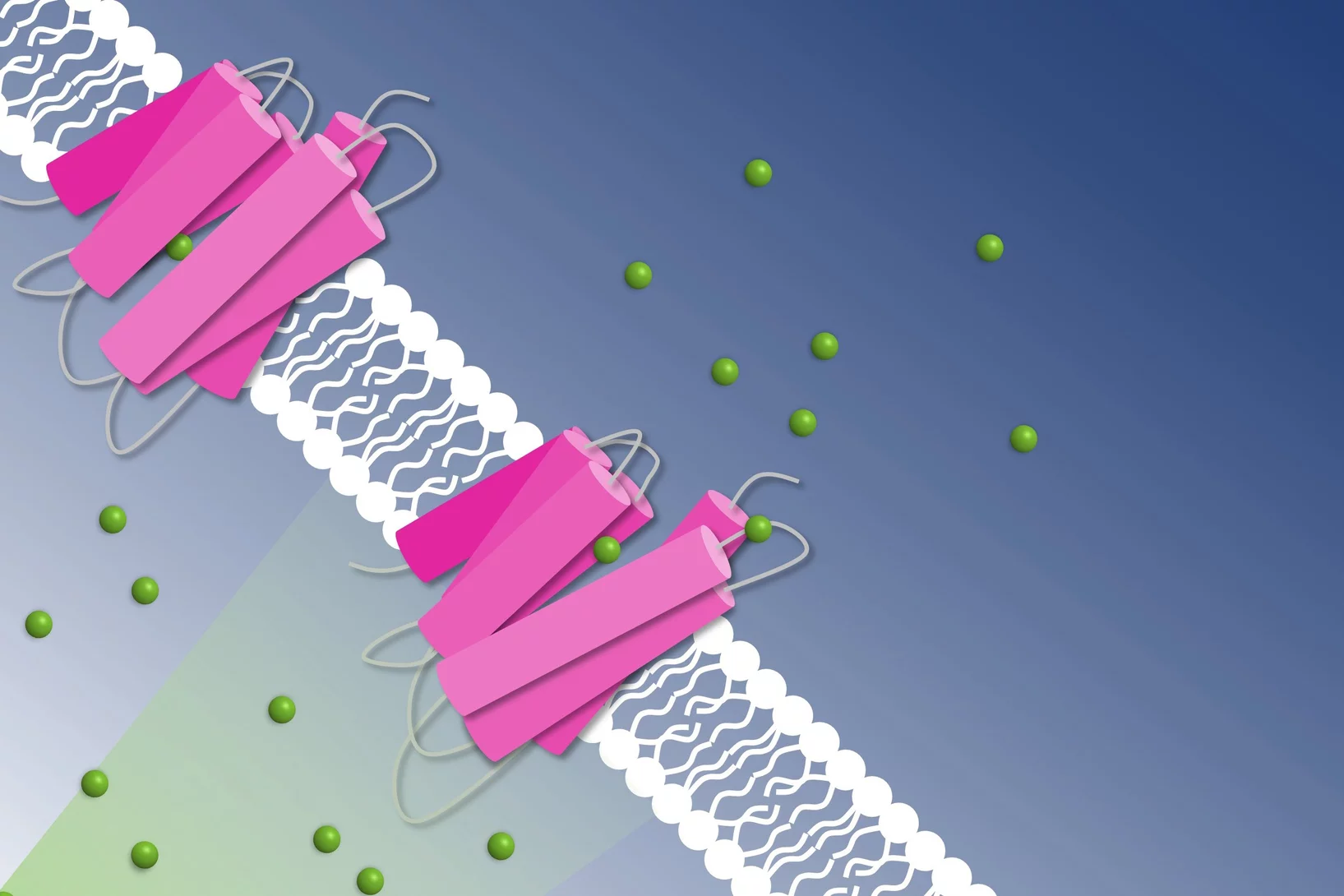
How to get chloride ions into the cell
A molecular movie shot at PSI reveals the mechanism of a light-driven chloride pump
EU XFEL Young Scientist Award for Camila Bacellar
Camila Bacellar, beamline scientist and group leader of the Alvra endstation at SwissFEL, has received the European XFEL Young Scientist Award. The award recognises the contribution of young scientists to research at the European XFEL.
Recent Publications
-
Garratt D, Das SK, Nelson KJ, Harich J, Freibert A, Bacellar C, et al.
Influence of substitution pattern on the dynamics of internal conversion and intersystem crossing in thiopyridone isomers
Physical Chemistry Chemical Physics. 2025; 27(31): 16371-16382. https://doi.org/10.1039/d5cp01456e
DORA PSI -
Gorel A, Shoeman RL, Hartmann E, Nizinski S, Appleby MV, Beale EV, et al.
Testing the limits: serial crystallography using unpatterned fixed targets
IUCrJ. 2025; 12(6): 12 (18 pp.). https://doi.org/10.1107/S2052252525008371
DORA PSI -
Knurr J, Hemberger P, Ascher P, Augustin S, Hoffman DJ, Knopp G, et al.
Ultrathin liquid sheets: water gets in shape for VUV absorption
Physical Chemistry Chemical Physics. 2025; 27(13): 6457-6464. https://doi.org/10.1039/d4cp04619f
DORA PSI