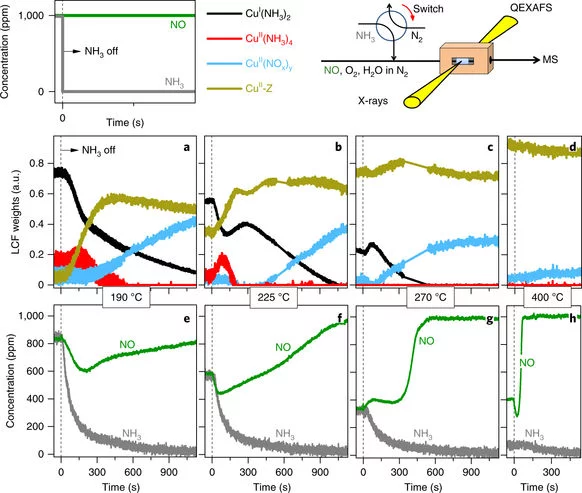
Practical catalysts often operate under dynamic conditions of temperature variations and sudden changes of feed composition that call for understanding of operation and catalyst structure under analogous experimental conditions. For instance, the copper-exchanged small-pore SSZ-13 catalyst used currently in the selective catalytic reduction of harmful nitrogen oxides from the exhaust gas of diesel-fuelled vehicles operates under recurrent ammonia dosage. Here, we report the design of unsteady state experiments that mimic such a dynamic environment to obtain key mechanistic information on this reaction. Through the combination of time-resolved X-ray absorption spectroscopy and transient experimentation, we were able to capture an ammonia inhibition effect on the rate-limiting copper re-oxidation at low temperature. The practical relevance of this observation was demonstrated by optimization of the ammonia dosage on a catalyst washcoat on cordierite honeycomb, resulting in lower ammonia consumption and an increase in nitrogen oxide conversion at low temperature.
Additional information
Link to the media releaseContact
Dr Maarten NachtegaalSuperXAS beamline
Laboratory for Synchrotron Radiation and Femtochemistry (LSF)
Swiss Light Source, Paul Scherrer Intitute
5232 Villigen-PSI, Switzerland
Telephone: +41 56 310 30 56
E-mail: marten.nachtegaal@psi.ch
Original Publication
Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13Adrian Marberger, Andrey W. Petrov, Patrick Steiger, Martin Elsener, Oliver Kröcher, Maarten Nachtegaal & Davide Ferri
Nature Catalysis, 8 March 2018
DOI: 10.1038/s41929-018-0032-6