The Operando X-ray Spectroscopy belongs to Environment Division (ENE) of PSI and is within a matrix organization responsible for the operation of the SuperXAS and Debye beamlines to LSF.
Scientific Highlights
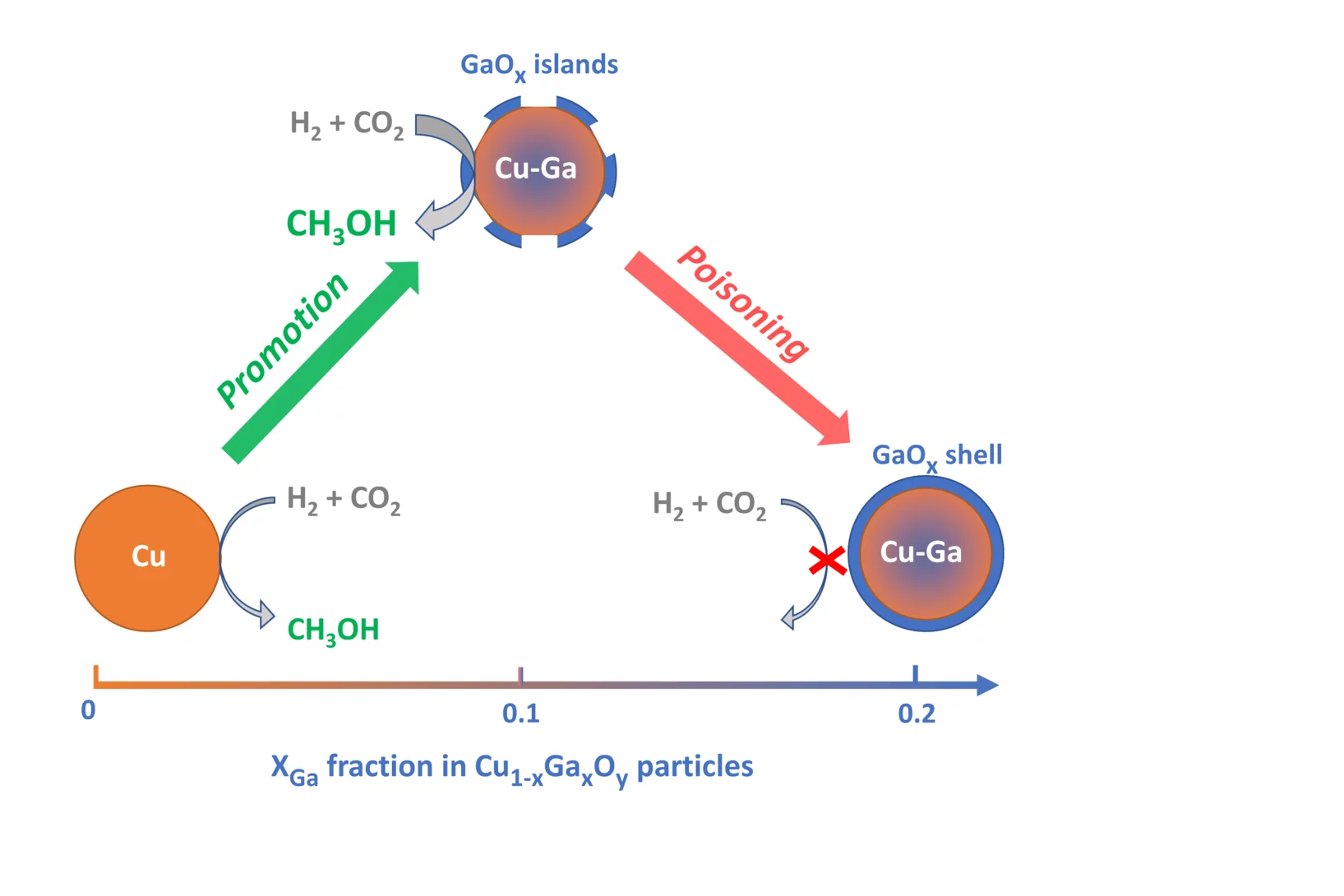
Promotion versus Poisoning in Copper–Gallium-Based CO2-to-Methanol Hydrogenation Catalysts
Cu–Ga-based CO2-to-methanol hydrogenation catalysts display a range of catalytic performance, depending on their preparation. Here, we investigated how the Ga/Cu ratio and Ga speciation affect the catalytic activity. Using surface organometallic chemistry, we prepared a series of silica-supported 3–6 nm Cu1–xGaxOy nanoparticles with a range of xGa. The materials display a volcano-type activity behavior, where methanol formation is promoted when xGa < 0.13–0.18 and is suppressed at higher values, indicating a poisoning of the catalysts. In situ X-ray absorption spectroscopy and in situ infrared spectroscopy helped to understand the structure-activity relationship.
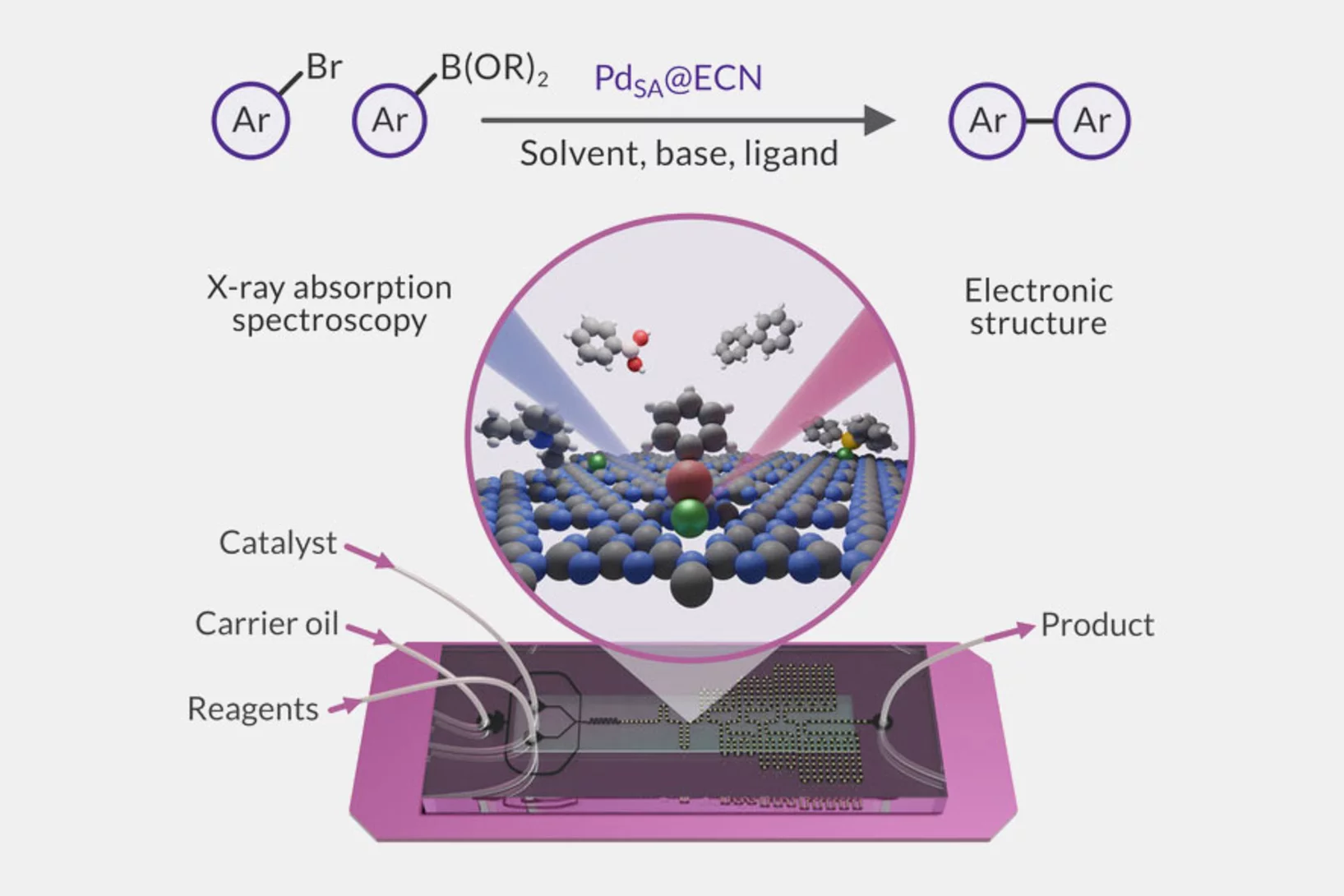
Microfluidic platform for in situ characterization of heterogenous catalysts
A deep understanding of active site architectures during surface-catalyzed reactions is a crucial step for the design of recyclable heterogeneous catalysts for organic synthesis. In this work, a droplet-based microfluidic setup was developed and applied to perform Suzuki-Miyaura coupling over heterogenous single-atom Pd-catalyst.
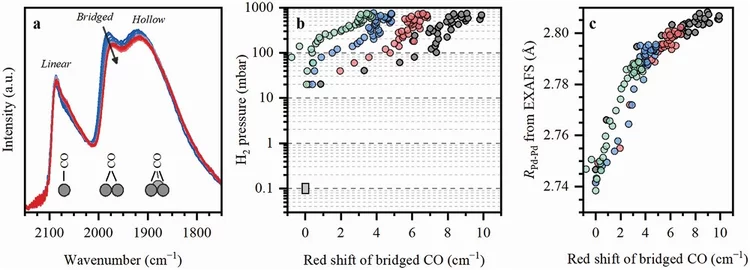
Machine Learning for Quantitative Structural Information from Infrared Spectra: The Case of Palladium Hydride
Infrared spectroscopy (IR) is a widely used technique enabling to identify specific functional groups in the molecule of interest based on their characteristic vibrational modes or the presence of a specific adsorption site based on the characteristic vibrational mode of an adsorbed probe molecule. The interpretation of an IR spectrum is generally carried out within a fingerprint paradigm by comparing the observed spectral features with the features of known references or theoretical calculations. This work demonstrates a method for extracting quantitative structural information beyond this approach by application of machine learning (ML) algorithms.