Show filters
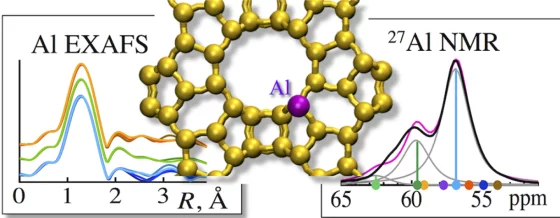
Aluminium sichtbar gemacht
PSI-Forschende haben in Zeolithen erstmals die genaue Lage der Aluminiumatome bestimmt, welche die Materialien zu so guten Katalysatoren machen.
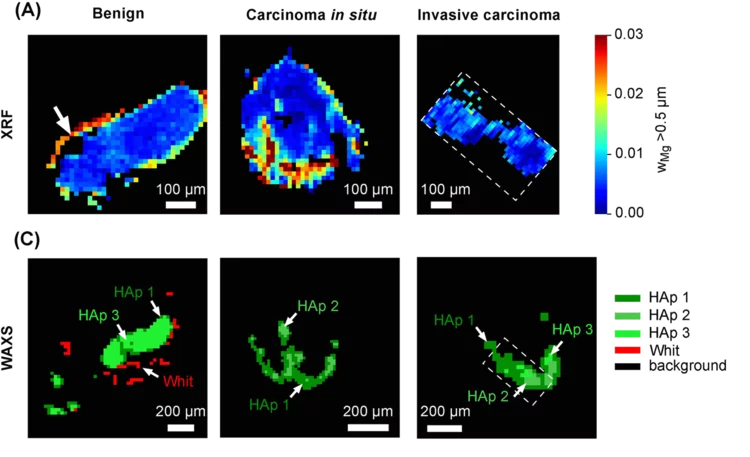
Whitlockite in mammary microcalcifications is not associated with breast cancer
Microcalcifications, small deposits of calcium-containing minerals that form in breast tissue, are often, but not always, a warning sign of breast cancer. The relationship between microcalcifications and cancer has not been fully understood thus far. Researchers discovered now that the relationship between microcalcifications and tumors seems to be linked to the presence of a particular mineral called whitlockite, which is rich in magnesium and is found in microcalcifications only in the absence of tumors.
Tender X-rays show how one of nature’s strongest bonds breaks
Short flashes of an unusual kind of X-ray light at SwissFEL and SLS bring scientists closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical.
Geheimnis der Stradivari-Geigen enthüllt
Wie ein internationales Team von Forschenden herausfand, griffen die alten italienischen Meister Stradivari und Guarneri beim Geigenbau zu unerwarteten chemischen Hilfsmitteln.
Insights into the world’s oldest pile carpet
High-resolution XRF imaging of the specific metal distribution within wool fibers at the PHOENIX beamline gives insights into traditional oriental dyeing procedures.
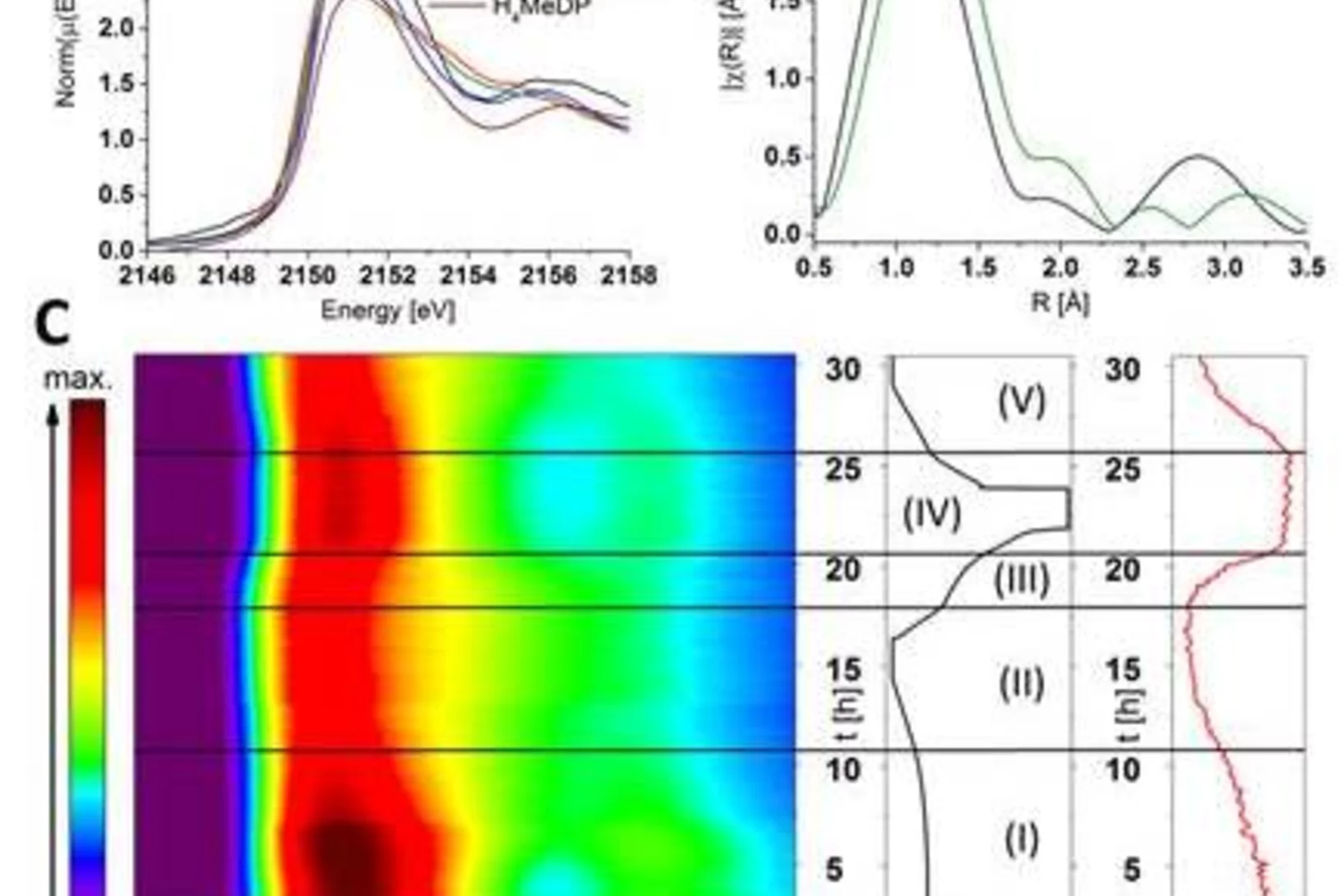
Phosphorus recovery from wastewater: Nitrogen K-edge micro-XANES spectroscopy unravels the effects of nitrification inhibitor on fertilizer phosphorus uptake of maize
Phosphorous containing fertilizers are essential to feed the growing population on earth. Because phosphorus (P) is a scarce resource in the European Union, recovering P from wastewater and sewage sludge has become extremely important. However, the availability of P to the plant is limited in such recycling P fertilizers. To overcome this problem, co-fertilization with nitrogen (N) in the form of ammonium and nitrification inhibitors, is a promising pathway. By applying the novel N K-edge micro-XRF and micro-XANES methods at the PHOENIX beamline on the soils, we could verify that a nitrification inhibitor indeed promotes ammonium fixation in fertilized soils, and hence causing a slow-release of temporarily fixed ammonium. This deceases local pH, making P better available to plants.
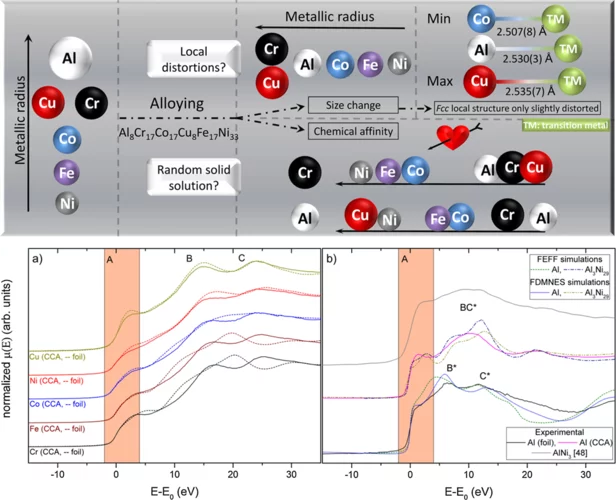
Uncovering the short range order and local distortions in high entropy alloys using EXAFS
High entropy alloys (HEA), medium entropy alloys and multi-phase compositionally complex alloys (CCA) have gained much attention in the last 20 years because of their outstanding mechanical properties. Such baseless alloys provide different open questions on local chemical ordering, lattice distortions, orbital hybridization and/or charge transfer which define the very nature of alloys’ mechanical properties. By combining EXAFS measurements in the rarely served tender x-ray range (PHOENIX-SLS, Al K-edge) and at higher X-ray energies (BM08-ESRF, transition metal K-edges), local chemical ordering in a CCA, Al8Cr17Co17Cu8Fe17Ni33 was quantified showing preferred Al-Ni and Al-Cu pairs. In addition, slight structural distortions, much lower than the predicted ones of metallic radii, were found.
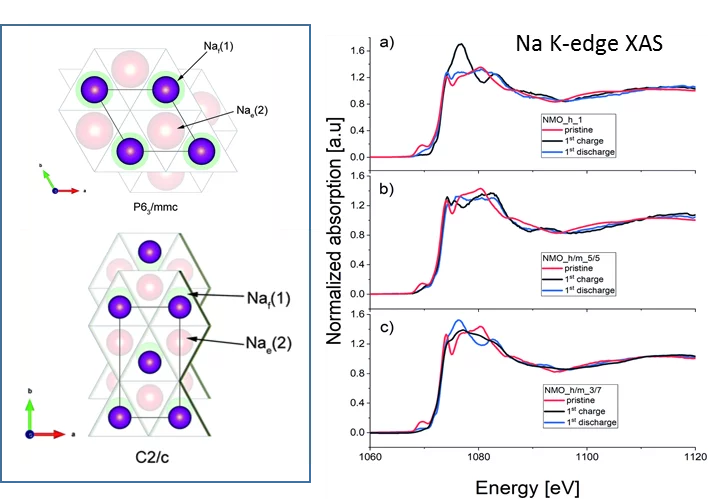
Sodium-ion batteries: a study of the structural and electrochemical properties of the layered cathode material NaxMnyO2
Being able to replace Lithium by the much more abundant sodium for new batteries would be an important asset for energy storage. For example, NaxMnyO2 cathodes would offer a high initial specific charge and a relatively high working potential. Despite long, intensive research of the electrochemical properties of these materials, the open key question remains unresolved: Where does the sodium goes to in the charging /discharging process. Unfortunately, the (de)sodiation mechanism in those materials was not completely understood, especially in terms of types of phases in which Na stays during cycling, which in turn impeded the optimization of its performance. Using the unique tender energy range of the PHOENIX beamline, we used Na K-edge X-ray absorption spectra measurements to gain a better understanding about the Na atomic positions in phases appearing during cycling. Thanks to this unique method, we established that observed high capacity in NaxMnyO2 is due to the high-voltage phase being an intergrowth structure between P2 and O2 type phases were Na ions stays both in tetrahedral and octahedral sites.
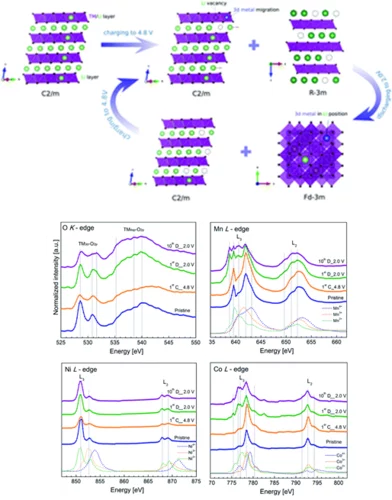
Lithium-ion batteries: following the redox reaction of oxygen and transition metals in the Li1.2Mn0.6Ni0.1Co0.1O2 electrode using X-ray absorption spectroscopy
The new generation of cathode materials from the Li-rich NMC (nickel-manganese-cobalt) group are under constant investigation due to their extremely high energy densities resulting from redox reactions involving both transition metals and lattice oxygen. Although a lot of research has been done so far, the exact mechanism of lithium (de)insertion in those materials, especially the reactions involving redox reactions of lattice oxygen is still elusive. Due to the particular battery design the observed reactions starts at the surface of the electrode that contacts the electrolyte and, as the reaction continues, goes deeper into the bulk structure. In order to follow the reactions taking place in the Li-rich NMC materials we aimed to exactly distinguish and characterize the phase transitions taking place on the surface and within the bulk of the Li1.2Mn0.6Ni0.1Co0.1O2 electrode. To do so we used comprehensive XAS measurements at the PHOENIX beamline, taking advantage of the unique options to perform in situ experiments in the soft energy range to study both the Oxygen K edge and the L edges of Ni, Co and Mn.
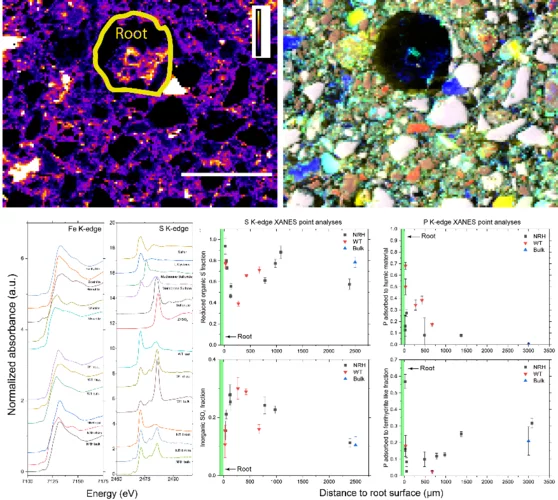
Root induced soil deformation influences Fe, S and P: rhizosphere chemistry investigated using synchrotron XRF and XANES
Taking up nutrients from the soil is key to plant growth. Understanding and potentially controlling this process is important when growing food but also when caring for natural habitats, which are the basis for life on Earth. Typically, nutrients are tightly chemically bound to the soil, and roots need to create a chemical environment to harvest nutrients. Here we use the special capabilities X-ray microscopy with tender X-rays to study the chemical changes of sulfur, phosphorus, and iron in the vicinity of plant roots (rhizosphere). We can show that Fe is slightly reduced, S is increasingly transformed into sulfate (SO42−) and phosphorus (P) is increasingly adsorbed to humic substances in this enrichment zone around the root.
Refined diagnosis of the “concrete disease”
When bridges, dam walls and other structures made of concrete (cement and aggregates such as sand/gravel) are marked by map-like cracks after a few decades, the diagnosis is ASR (alkali-silica Reaction), in popular science terms also called “concrete disease or concrete cancer”. The ASR-induced microscale crack initiation can hardly be modelled, mainly due to our limited knowledge of the structure and property of the ASR products. Using X-ray absorption micro-spectroscopy at the PHOENIX beamline of the Swiss Light Source (SLS) allowed a refined diagnosis of ASR products by providing new insights into the crystallinity and structure of ASR products with micro-scale resolution.
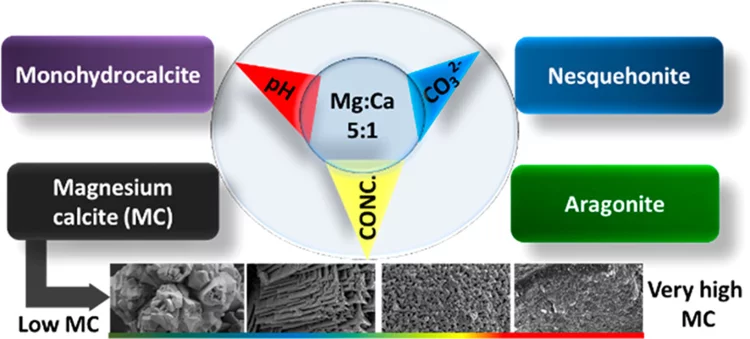
Tuning the magnesium content in magnesium rich-calcites
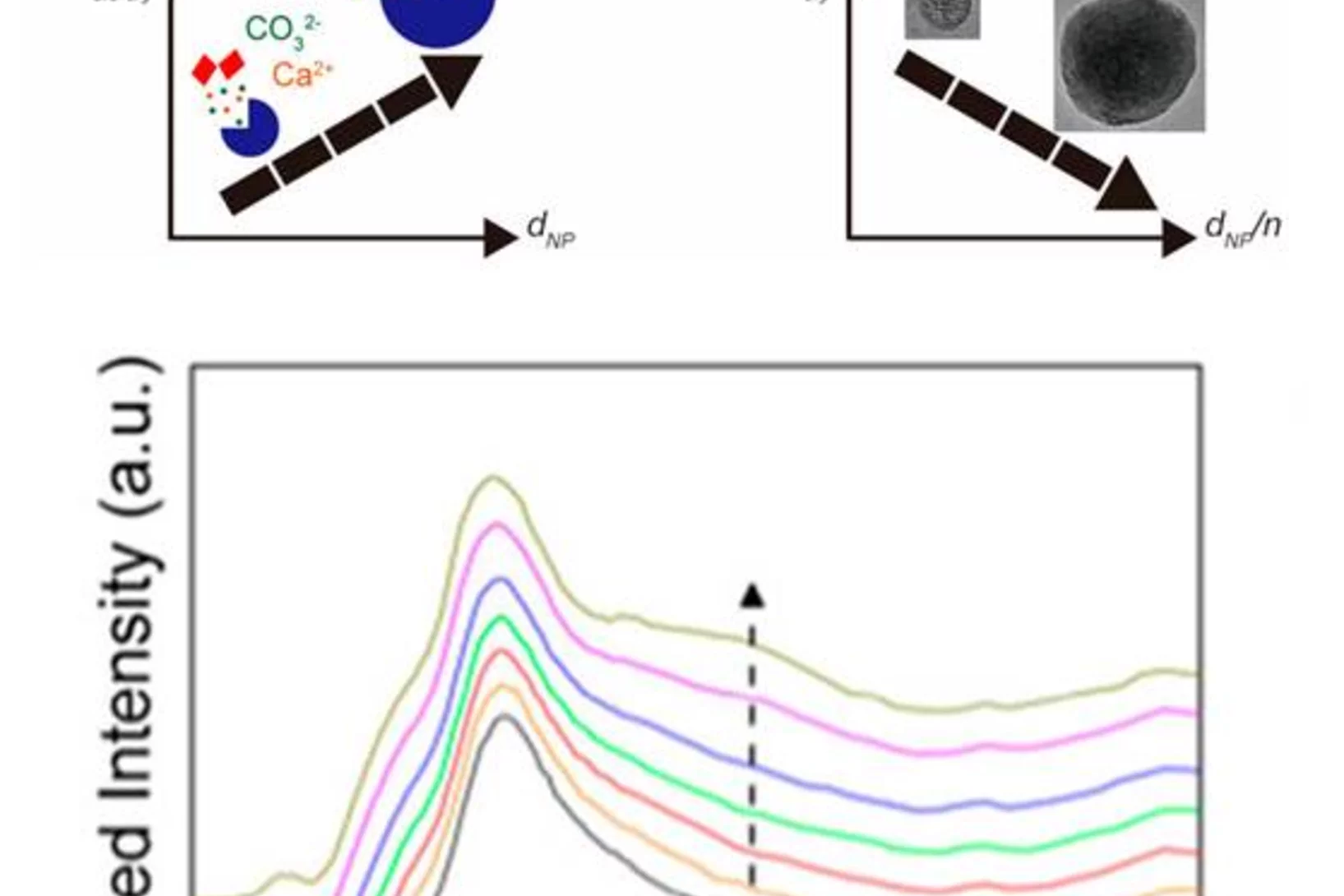
Magnesium rich calcites are important functional biominerals. For example, they can be found in protective shells or eye lenses. Natural organism provide a surprisingly high degree of control on the amount of magnesium incorporation into calcites by yet not well understood mechanisms. Understanding such control mechanism is important when designing bio inspired functional materials. Here we systematically explore the impact of thermodynamic parameters on the degree of magnesium incorporation into calcite. In particular, we identify the thermodynamic conditions, where very high magnesium rich calcites (50% Mg/50% Ca) forms under ambient conditions of temperature and pressure. This is an important finding for geochemistry: Very high magnesium rich calcite is believed to be the precursor for dolomite. Despite its frequent occurrence in nature, its unknown formation pathway remains one of the big mysteries in geochemistry.
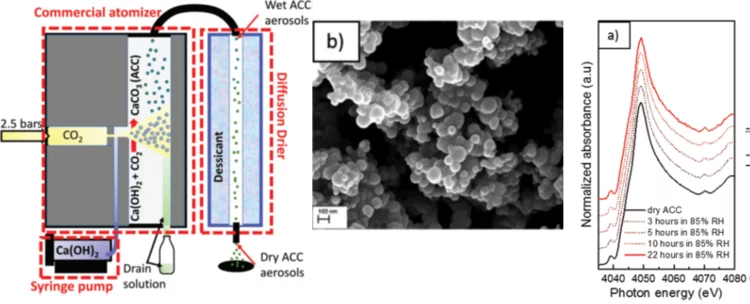
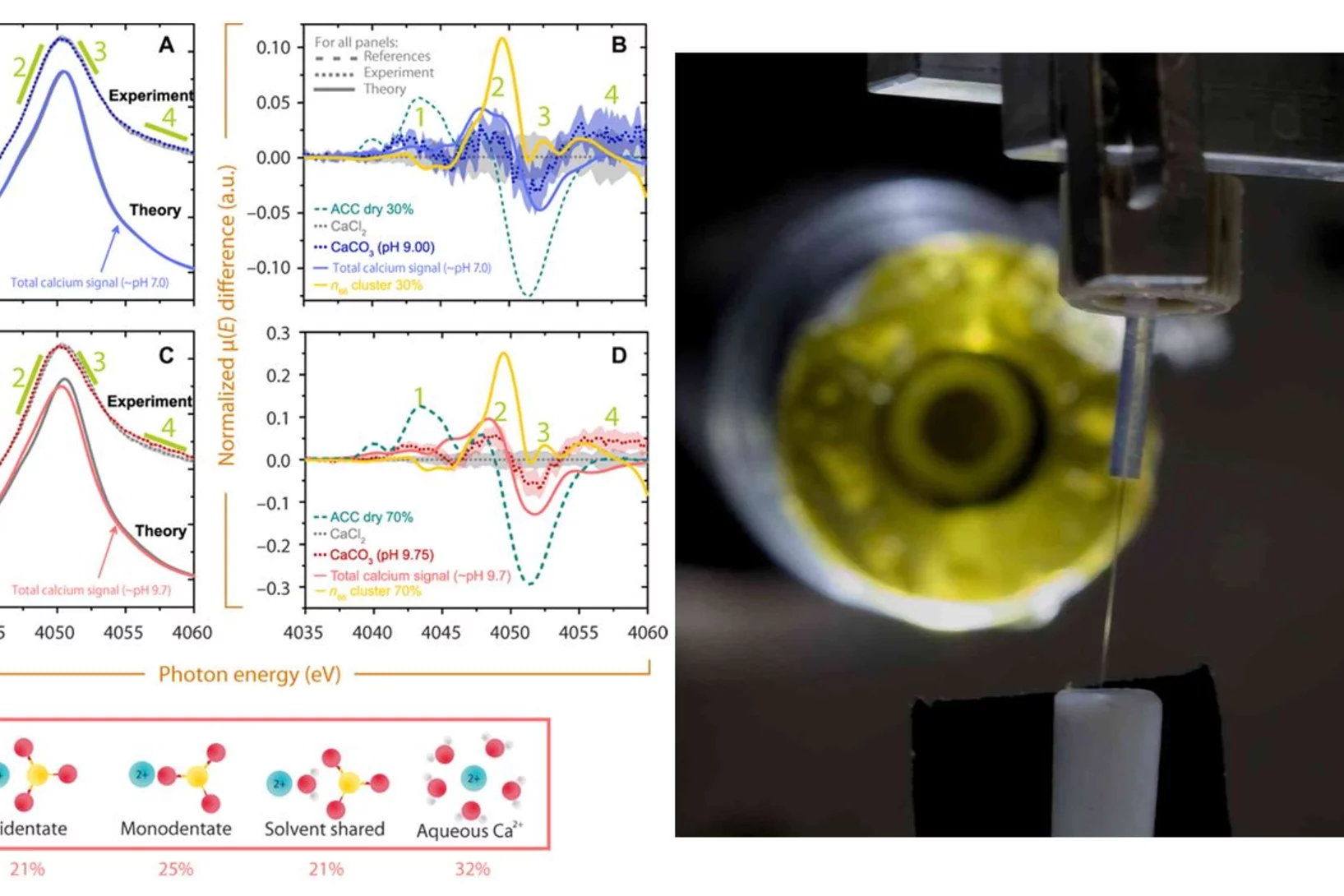
Aerosol-based synthesis of pure and stable amorphous calcium carbonate
Calcium carbonates are key materials to biomineralization, they are frequently used in industrial applications and also for carbon capture technologies. Finally, they serve as an important model system to test novel nucleation theories. Calcium carbonate crystalizes in a multi-step process, where amorphous calcium carbonate (ACC) is the most important precursor in the crystallization process. Existing synthesis protocols generate ACC of different stability and purity. To improve our mechanistic understanding of carbonate crystallization, reactivity and polymorph formation, the reproducible synthesis of clean and stable ACC is an important, and yet unresolved step. Here we use the fast reaction of CO2 with calcium hydroxide in airborne aerosols to reproducibly create pure and stable ACC, which may serve as a well-defined starting material for further chemical processing.
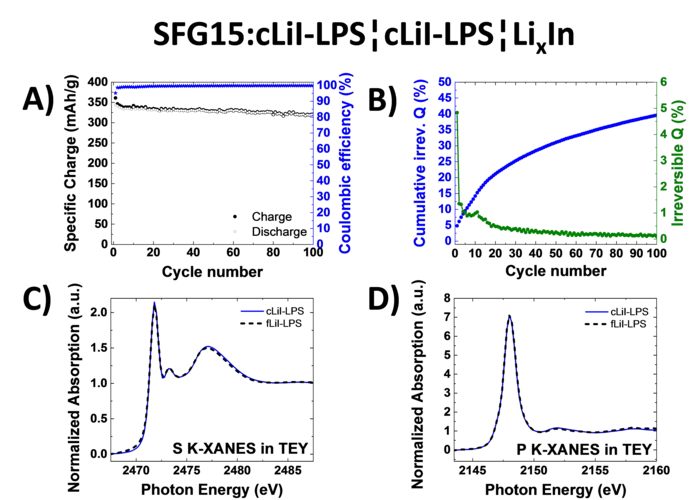
Study of Graphite Cycling in Sulfide Solid Electrolytes
Nowadays, most of the commercial Li-ion batteries employ graphite as the active material in negative electrodes. In the race for the next-generation Li-ion batteries, tremendous research efforts in academia and industry are carried out to replace the current flammable liquid electrolyte with a solid electrolyte, which could improve both, the batteries safety and energy density. Our study investigates two different sulfide-based solid electrolytes, 0.75Li2S-0.25P2S5 (LPS) and 0.3LiI-0.7(0.75Li2S-0.25P2S5), in combination with graphite and discloses the stability of the graphite-solid electrolyte interface. Optimizing the electrode morphology is the key to enhance the rate capability of all-solid-state cells. Using the special tender X-ray range allows chemical characterization of sulfur, phosphor and iodine.
HERCULES school 2019 at SLS
In the week of April 1-5 PSI welcomes 20 PhD students and postdocs taking part in the European HERCULES 2019 school on Neutron and Synchrotron Radiation. They will attend lectures and perform two days of practical courses at several beam lines of the Swiss Light Source.
Investigation of anionic redox activities in organic-based electrode for Li-ion batteries
To date the electrochemical activity of battery materials was always relying in the oxidation/reduction of cationic redox (change of oxidation state of transition metals generally). However, recently, it was established in new cathode materials (so call Li-rich cathode) that the oxygen from the crystal lattice might also play the role of anionic redox center leading to enhance then the specific charge of battery materials.
Von Hamos spectrometer for tender energies open to users
A new compact von Hamos spectrometer for tender x-rays (current energy range 2.25-4.5 keV), is now available for emission spectroscopy. This spectrometer allows analyzing the energetic composition of fluorescent light from the sample. It provides research opportunities for emission spectroscopy, and RIXS on the K (P-Sc), L (Zr-Cs) and M (Ir-Fr) absorption edges.
Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability
Carbonate minerals serve as reservoir for CO2 in the global CO2 cycle, as biomineral in animal skeletons and shells of marine animals, and are used in carbon capturing techniques. Moreover, they serve as an important model system in crystallization studies, and have important commercial applications, for example as fillers. Researchers from EPFL and PSI developed a new methodology to study the crystallization of CaCO3 that offers both high temporal and spatial resolution, which is the key challenge in elucidating early stages of crystallization. Using X-ray absorption spectroscopy and other techniques it could be demonstrated that the degree of hydration of amorphous CaCO3 increases during its growth. As a result of the increasing degree of hydration, the stability of the resulting amorphous particles against solid-state crystallization decreases.
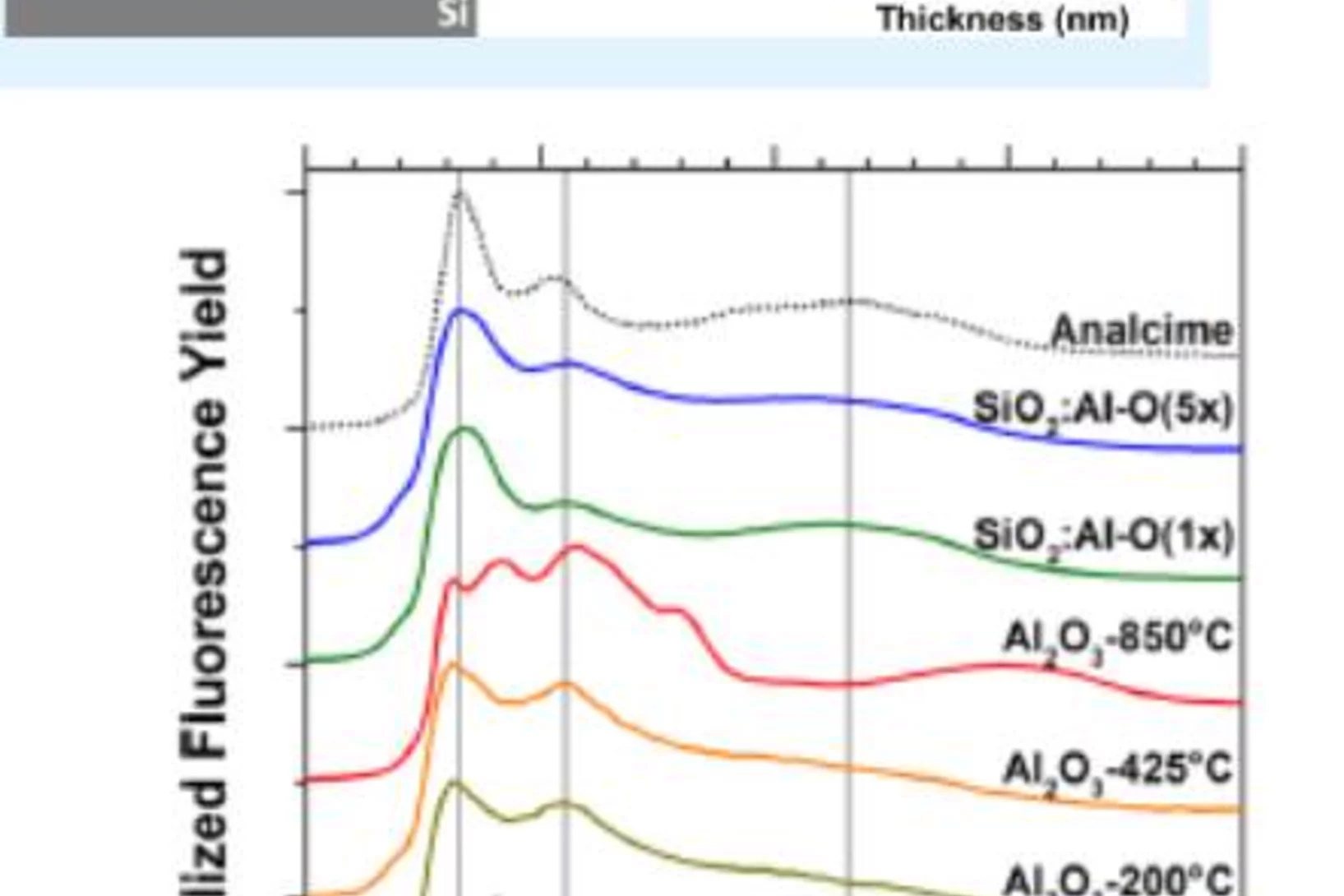
The negative charge density in Al-O monolayers on SiO2 surfaces
Single atomic layers of aluminum oxide embedded in SiO2 thin films, play an important role for the design of carrier-selective passivating contacts for high efficiency silicon based photovoltaic applications. Researchers from the Australian National University (ANU, Canberra, Australia), the Karlsruhe Institute of Technology (IT, Karlsruhe, Germany) and PSI have used synchrotron radiation to reveal the bonding configuration and local atomic surrounding of the Al-atoms in such surface oxide layers. The results corroborates theoretical calculations and contribute to a new model to explain the origin of the negative fixed charge in the Al-O/SiO2 stack, which has promising properties for a carrier-selective passivating contact for future silicon solar cells.
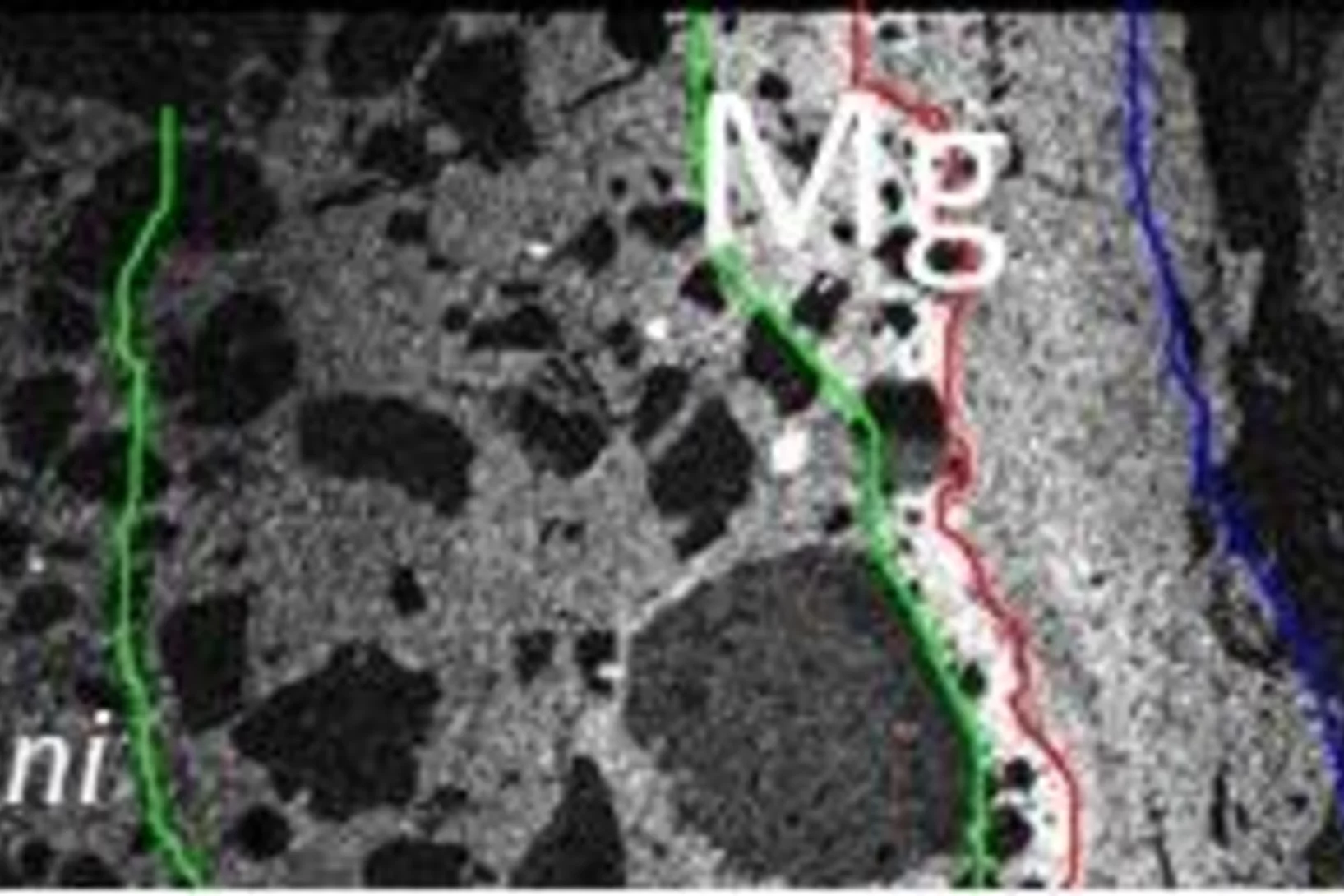
When man-made stones meet natural rocks – Shedding light on Mg-rich phases appearing at the interface between concrete and clay
Claystones and cement-based materials are key materials for safe disposal of radioactive waste in deep geological repositories. In Switzerland, Opalinus Clay, was selected as geological host material. At the Mont Terri rock laboratory the alteration of cement in contact with the natural clay is studied in a several years lasting experiment. The formation of different magnesium containing phases at the interface was studied using X-ray absorption micro-spectroscopy at the PHOENIX beamline of the Swiss Light Source (SLS).
HERCULES at the Swiss Light Source
In the week of March 18-23 PSI welcomes 20 PhD students and postdocs taking part in the HERCULES 2018 school on Neutron and Synchrotron Radiation. They will attend lectures and perform two days of practical courses at several beam lines of the Swiss Light Source.
Are supersaturated calcium carbonate solutions classical or non-classical ?
Classical theory predicts that supersaturated carbonate solutions consist mostly of ions and ion pairs, with a small number of larger clusters present in the solution. The population of the different sized clusters in a solution is solely defined by the cluster’s size dependent Free Energy. If clusters are large enough they serve as nucleation germs for a new solid phase. The nucleation occurs once the surface free energy barrier posed by the new solid-liquid interface is overcome by the free energy win from bulk phase growth.
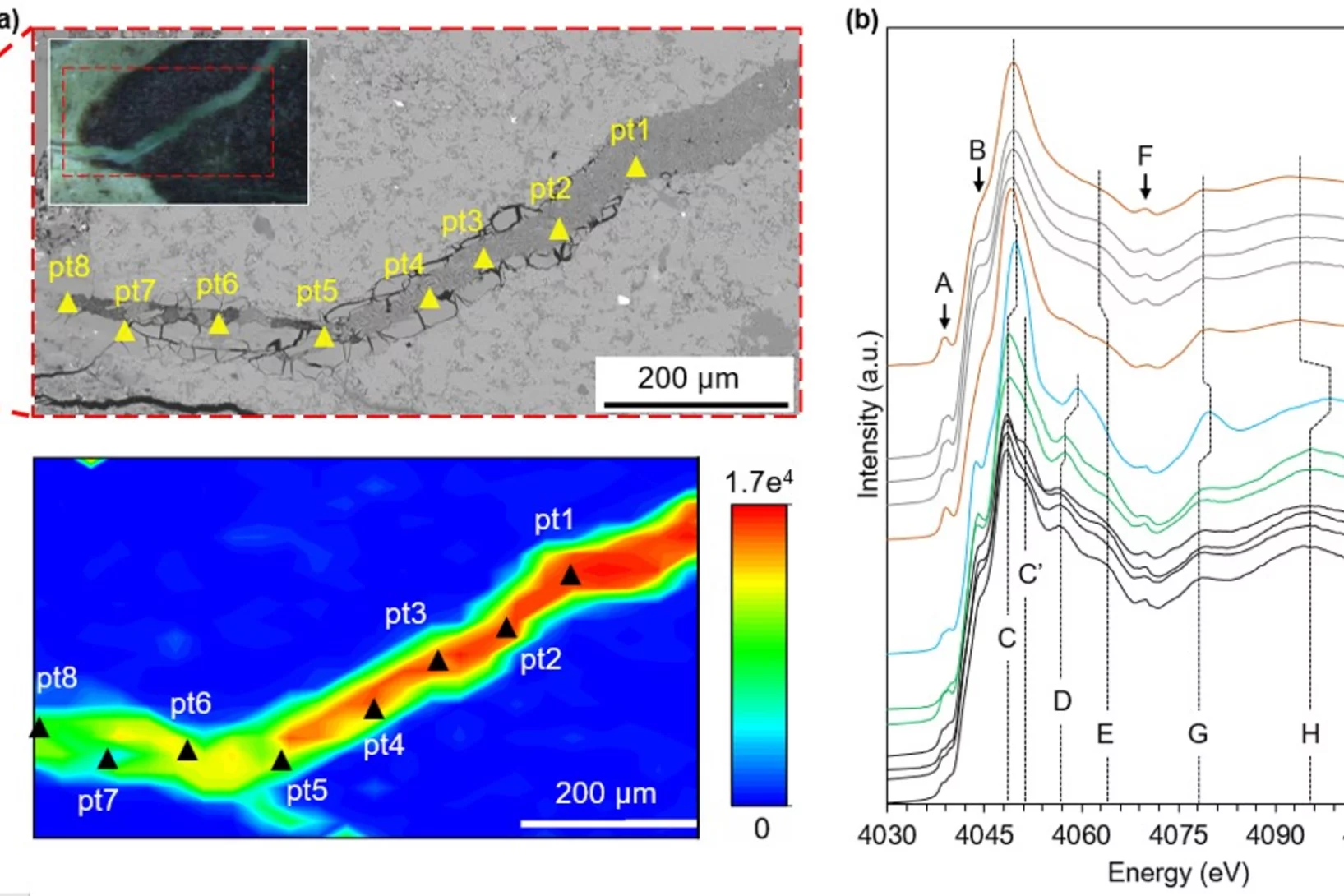
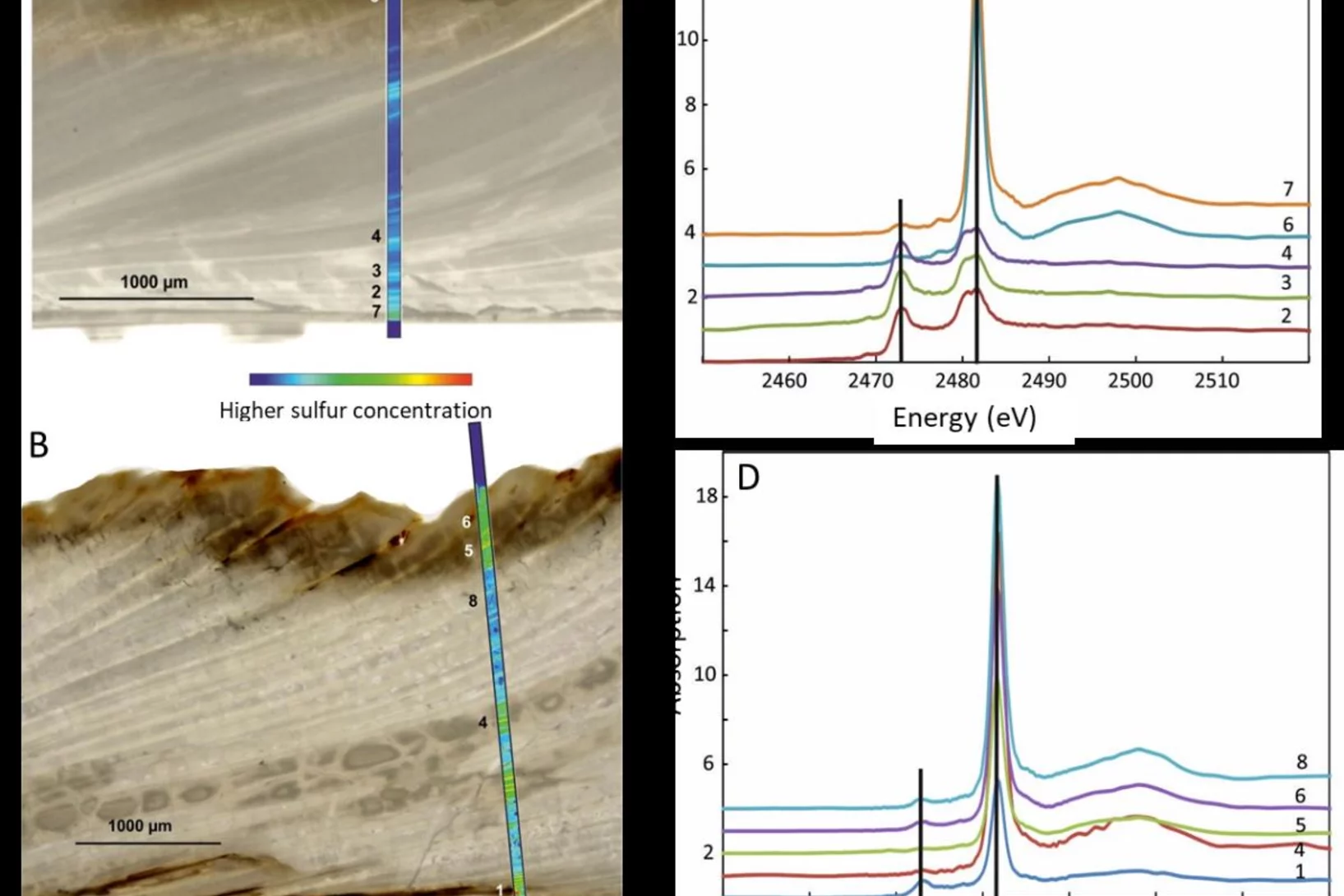
How is sulfur incorporated in biogenic carbonates and how is it affected by hydrothermal alteration?
Sulfate in biogenic carbonates is an important proxy for reconstructing the marine sulfur cycle. To investigate the exact location of carbonate associated sulfate (CAS) in biogenic carbonates and the effects of diagenetic alteration on sulfur in carbonates, shells of the marine bivalve Arctica islandica were artificially altered in modified seawater. Sulfur XANES analyses showed that CAS in A. islandica is indeed incorporated into the mineral part of the pristine shell, most likely as a hydrated or partly hydrated sulfate phase. The multi-analytical approach of XANES and µ-XRF analyses, sulfur isotope measurements, NanoSIMS analyses, and microstructural analysis on thin-sections of the shell samples further revealed that the different sulfur in a bivalve shell sensitively reacts to artificially induced hydrothermal alteration.
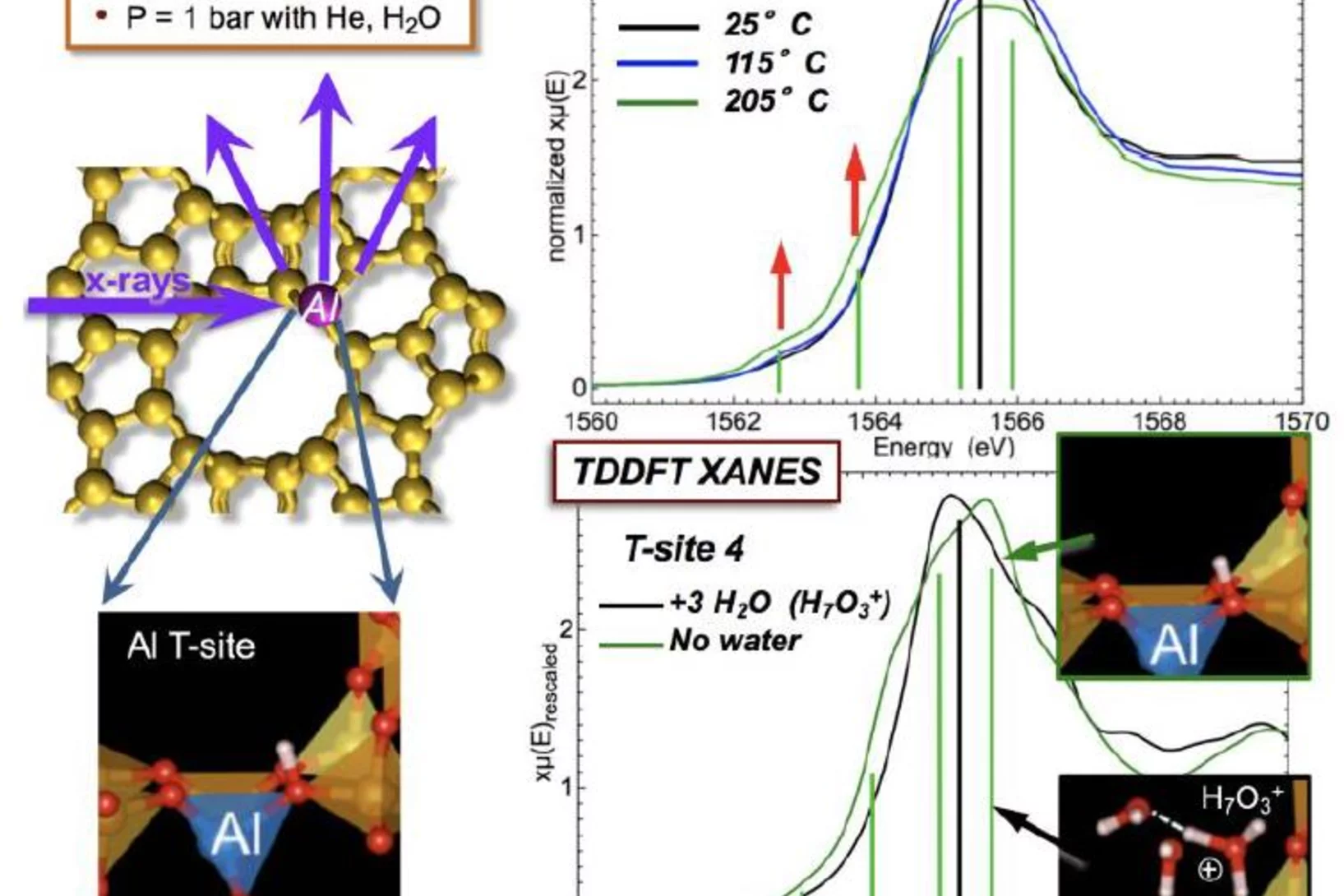
Tracking the Chemical Transformations at a Zeolite Brønsted Acid Site with Al K-edge XANES
Al T-sites are of crucial importance for the function of zeolite catalysts. These T-sites, which serve as Brønsted acid reaction centers, interact strongly with water. The location of these T-sites and their chemical state in the presence of water were elucidated using x-ray absorption spectroscopy (XAS) at the PHOENIX beamline at the Swiss Light Source of the PSI.
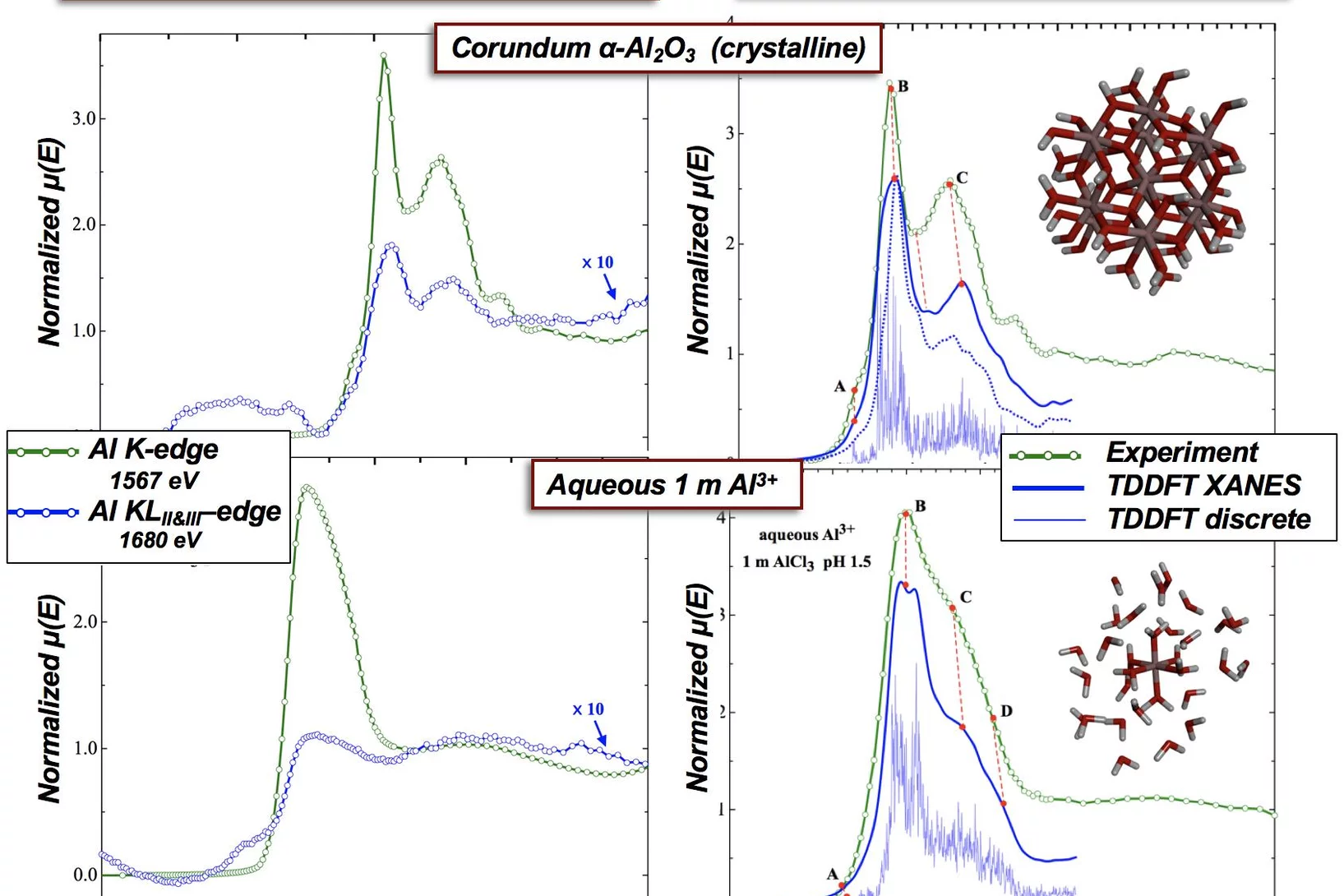
Single- (K) and Double-Electron Excitation (KLII&III) XANES Spectra of α-Alumina and Aqueous Al3+•(H2O)6
X-ray absorption spectroscopy (XAS) probes the local environment around an atom by study of the local photoelectron’s scattering. Multielectron excitations become more important at higher x-ray dose, which are used for examples in x-FEL experiments. Here we demonstrate that multielectron excitations, observed in the Al K-edges EXAFS spectra can be used to derive structural information.
Quantitatively Probing the Al Distribution in Zeolites
The degree of substitution of Si4+ by Al3+ in the oxygen-terminated tetrahedra (Al T-sites) of zeolites determines the concentration of ion-exchange and Brønsted acid sites. Because the location of the tetrahedra and the associated subtle variations in bond angles influence the acid strength, quantitative information about Al T-sites in the framework is critical to rationalize catalytic properties and to design new catalysts.