Description
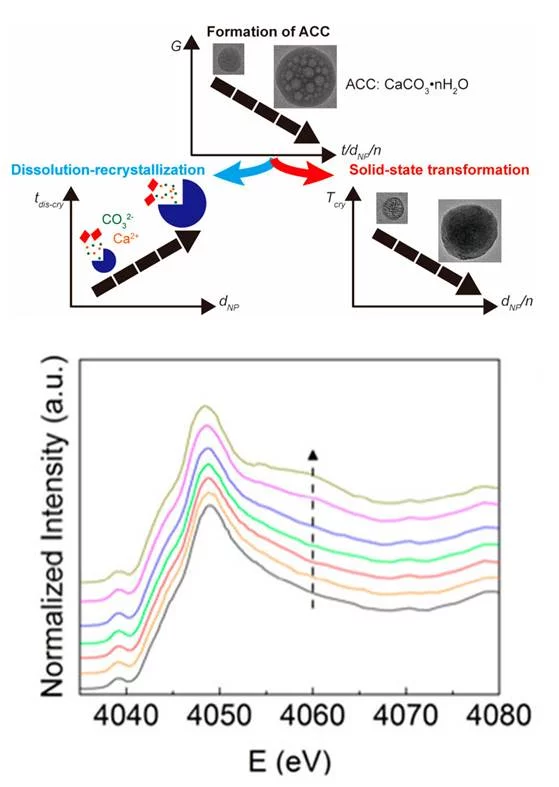
The mechanisms through which minerals such as CaCO3 form are still not fully understood, making it very difficult to control the structure and properties of the resulting crystals. This difficulty can, in parts, be assigned to the lack of characterization methods that allow studying the evolution of the formation of these minerals at early stages. Researchers from EPFL and PSI introduced a microfluidic spray-dryer that was developed at EPFL and enables quenching chemical reactions within milliseconds. This device thus opens up new possibilities to study the formation of minerals at early stages with a high temporal and spatial resolution. Using X-ray absorption spectroscopy, combined with electron microscopy, X-ray photoelectron spectroscopy, and infrared spectroscopy measurements, it could be shown that during early stages of the formation of amorphous CaCO3, their degree of hydration increases with increasing formation time. Hydrated amorphous CaCO3 is less stable against the solid-state transformations into its crystalline counterpart. Therefore, this finding implies that the crystallization transition temperature can be tuned towards lower temperatures by changing the formation time. This implication was experimentally validated. Moreover, the size of the resulting crystalline grains increases with increasing degree of hydration, most likely because the mobility of the ions during their re-arrangement into crystals is higher. This finding opens up new possibilities to design CaCO3-based materials with a superior control over their structure and hence their properties.
Contact
Prof. E. Amstad and/or Mr. H. DuInstitute of Materials
Soft Materials Laboratory
Ecole Polytechnique Federale de Lausanne (EPFL)
1015 Lausanne, Switzerland
Telephone: +41 21 69 32958
E-mail: esther.amstad@epfl.ch
Dr. Thomas Huthwelker
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Original Publication
Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and StabilityHuachuan Du, Mathias Steinacher, Camelia Borca, Thomas Huthwelker, Anna Murello,Francesco Stellacci, and Esther Amstad
J. Am. Chem. Soc. 2018, 140, 14289−14299
DOI: 10.1021/jacs.8b08298