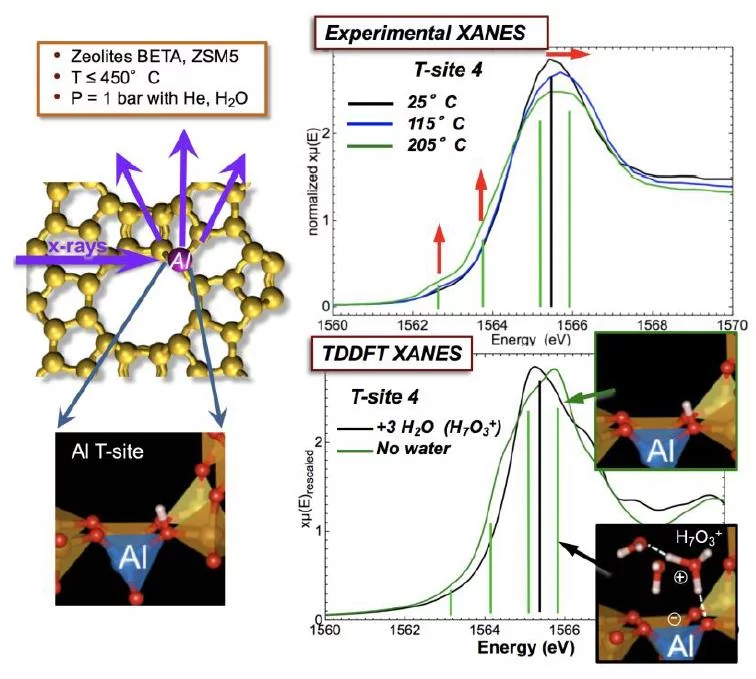
The Brønsted acid T-site is a primary reactive center underlying a broad range of zeolite chemistry. The structural changes induced by reversible formation of Brønsted acidic sites and hydronium ions with water in a zeolite with MFI structure are detected using Al K-edge XANES spectroscopy. In the presence of an ample concentration of water, the protons are present as hydrated hydronium ions (H3O+(H2O)n) that are ion-paired to the zeolite. Loss of water molecules hydrating the hydronium ions leads to an unstable free hydronium ion that dissociates to form the hydroxylated T-site. The formation of this SiOHAl species leads to the elongation of one of the four Al–O bonds and causes significant distortion of the tetrahedral symmetry about the Al atom. This distortion leads to the appearance of new pre-edge features in the Al K-edge X-ray absorption near edge structure (XANES) spectra. The pre-edge peak assignment is confirmed by time-dependent density functional (TDDFT) theory calculation of the XANES spectrum. The XANES spectra are also sensitive to solutes or solvents that are in proximity to the T-site. As temperature increases, the minor fraction of extra-framework Al present in the sample at ambient conditions in octahedral coordination is converted to tetrahedral coordination through the decoordination of H2O ligands.
Contact
Dr. John L. FultonStaff Scientist, Physical Sciences Division
Pacific Northwest National Laboratory
Richland, Washington, USA
E-mail: john.fulton@pnnl.gov
Dr. Niranjan Govind
Computational Scientist, Environmental and Molecular Sciences Division
Pacific Northwest National Laboratory
Richland, Washington, USA
E-mail: niri.govind@pnnl.gov
Dr. Thomas Huthwelker
Swiss Light Source
Forshungsstrase 111
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Original Publications
Tracking the Chemical Transformations at the Brønsted Acid Site upon Water-Induced Deprotonation in a Zeolite PoreAleksei Vjunov, Meng Wang, Niranjan Govind, Thomas Huthwelker, Hui Shi, Donghai Mei, John L. Fulton, and Johannes A. Lercher
_Chem. Mater., vol. 29 (21), pp 9030–9042 _, Oct 2017
DOI: 10.1021/acs.chemmater.7b02133
Quantitatively Probing the Al Distribution in Zeolites
Aleksei Vjunov, John L. Fulton, Thomas Huthwelker, Sonia Pin, Donghai Mei, Gregory K. Schenter, Niranjan Govind, Donald M. Camaioni, Jian Zhi Hu, and Johannes A. Lercher
_J. Am. Chem. Soc., vol 136 (23), pp 8296–8306 _, May 2014
DOI: 10.1021/ja501361v