The nature of high activity of Cu/ZnO catalyst in methanol synthesis remains the subject of intensive debate. Here, the authors are revisiting carbon dioxide hydrogenation mechanism using high-pressure operando techniques.
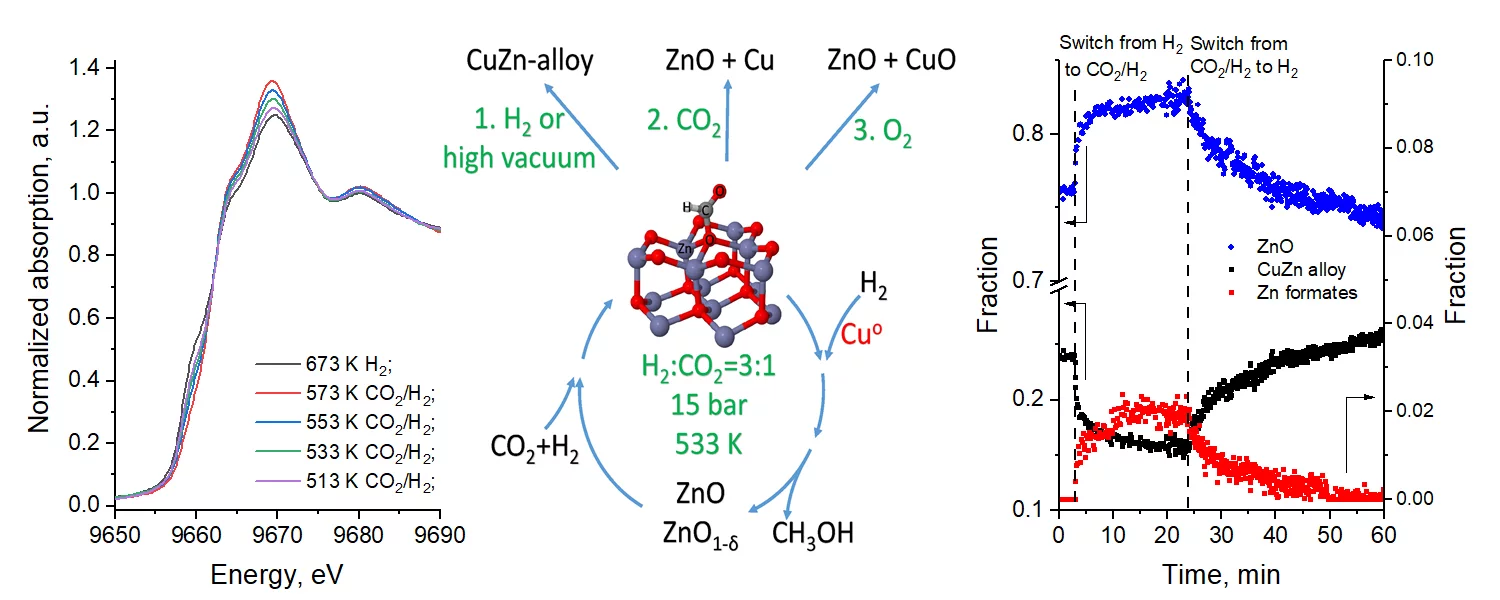
In spite of numerous works in the field of chemical valorization of carbon dioxide into methanol, the nature of high activity of Cu/ZnO catalysts, including the reaction mechanism and the structure of the catalyst active site, remains the subject of intensive debate. By using high-pressure operando techniques: steady-state isotope transient kinetic analysis coupled with infrared spectroscopy, together with time-resolved X-ray absorption spectroscopy and X-ray powder diffraction, and supported by electron microscopy and theoretical modeling, we present direct evidence that zinc formate is the principal observable reactive intermediate, which in the presence of hydrogen converts into methanol. Our results indicate that the copper–zinc alloy undergoes oxidation under reaction conditions into zinc formate, zinc oxide and metallic copper. The intimate contact between zinc and copper phases facilitates zinc formate formation and its hydrogenation by hydrogen to methanol.
Original Publication
The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol
M. Zabilskiy , V.L. Sushkevich, D. Palagin, M.A. Newton, F. Krumeich, J.A. van Bokhoven,
Nature Communications, 15 May 2020
DOI: 10.1038/s41467-020-16342-1