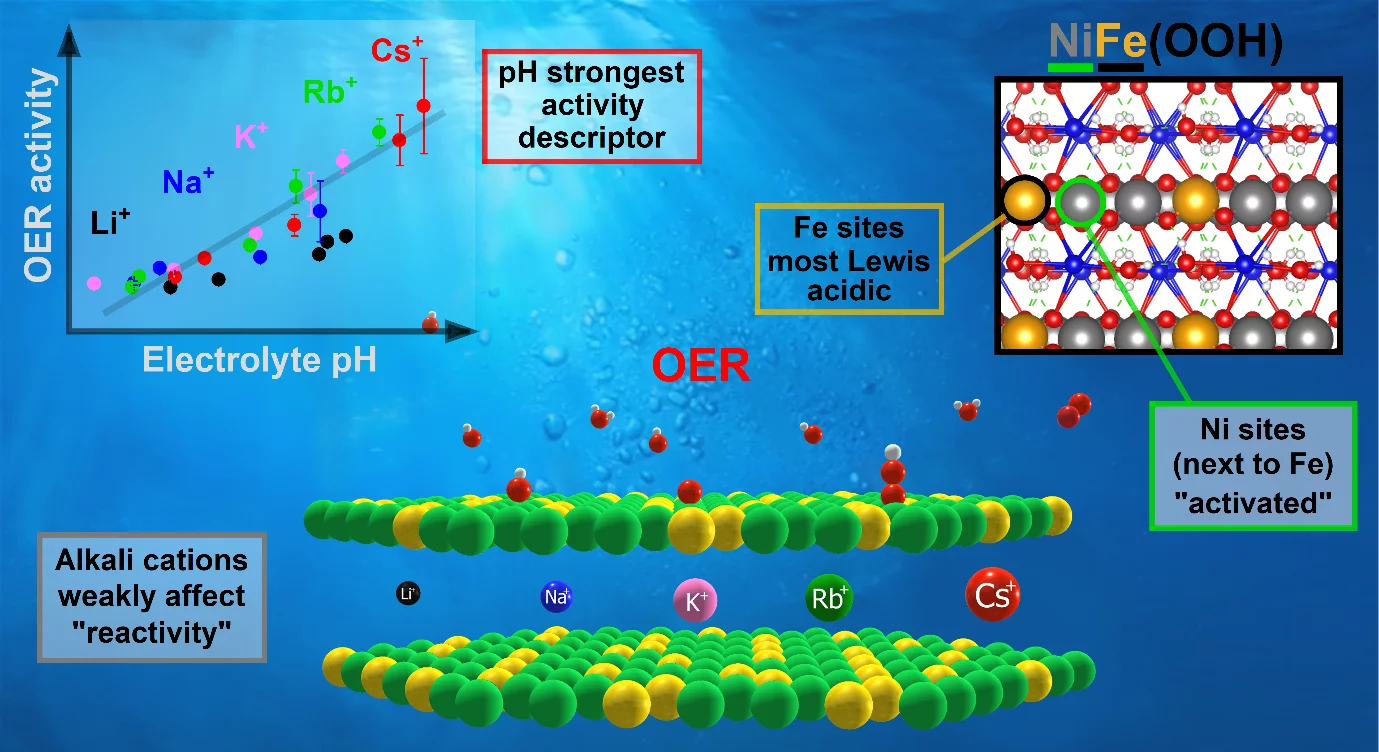
Ni-Fe oxyhydroxide is among the most active oxygen evolution electrocatalysts. Electrolyte alkali metal cations modify the activity and reaction intermediates, however, the exact mechanism is at question due to unexplained deviations from the cation size trend. Our X-ray absorption spectroelectrochemical results show that the OER activity follows the variations in .electrolyte pH rather than a specific cation. Our DFT-based reactivity descriptors confirm the conclusions of an indirect pH effect.
To slow down the growth of the steadily increasing carbon footprint, a transition to renewable energy is imperative. Electrochemical water splitting (2 H2O → 2 H2 + O2) offers a zero-carbon route to hydrogen (H2) from water, where the main cause of energy loss and cost in this process is the anodic oxygen evolution reaction (OER). To make water a viable source for H2, efforts therefore need to be focused on more efficient OER electrocatalysts. Efficient oxygen evolution reaction (OER) electrocatalysts are pivotal for sustainable fuel production, where the Ni-Fe oxyhydroxide (OOH) is among the most active catalysts for alkaline OER. Here, we investigate the OER activity and redox-activity using X-ray absorption spectroscopy (XAS) of a Ni–Fe oxyhydroxide (OOH) electrocatalyst in the presence of alkali metal cations (Li+, Na+, K+, Rb+, Cs+), which is one of the best performing catalysts in alkaline media.
In conclusion, we demonstrate that the effect of alkali metal cations on the OER activity of the Ni65Fe35(OOH) catalyst can be explained by differenes in the electrolyte pH, supported by the directional shift of the Ni2+→Ni3+/4+ redox-peak and the OER activity. Since the basicity increases in the order LiOH < NaOH < KOH < RbOH < CsOH following the cation size (explained by their pKb values), the electrolyte pH increases accordingly from small to large alkali metal cations due to an increase in the amount of dissociated OH−. Since the OER activity is sensitive to pH, this is able to explain previously puzzling discrepancies between the trend in cation size and the OER activity, since any factor introducing uncertainties in the OH− concentration (or electrolyte concentration) can affect the OER activity. Our DFT-derived reactivity descriptors further support that alkali metal cations do not significantly alter the intrinsic reactivity properties of either Ni, Fe, or O lattice in contrast to Fe substitution, which instead increases the Lewis acidity of neighboring Ni-sites (although Fe remains the most Lewis acidic site). The alkali cations are therefore unlikely to account for the differences in the OER activity. Thus, our DFT results are in line with the experimental findings that the electrolyte pH constitutes the strongest activity descriptor. Future studies therefore need to explore possible pH-effects in oxide-derived catalysts in striving for understanding the role of alkali metal cations in OER electrocatalysis.
Contact
Dr Olga Safonova
SuperXAS beamline
Operando spectroscopy group & Laboratory for Synchrotron Radiation and Femtochemistry (LSF)
Swiss Light Source, Paul Scherrer Intitute
5232 Villigen-PSI, Switzerland
+41 56 310 58 05
olga.safonova@psi.ch
Original Publication
Key activity descriptors of nickel-iron oxygen evolution electrocatalysts in the presence of alkali metal cations
Görlin M, Halldin Stenlid J, Koroidov S, Wang H-Y, Börner M, Shipilin M, Kalinko A, Murzin V, Safonova OV, Nachtegaal M, Uheida A, Dutta J, Bauer M, Nilsson A & Diaz-Morales O
Nature Communications, 02 December 2020
DOI:10.1038/s41467-020-19729-2