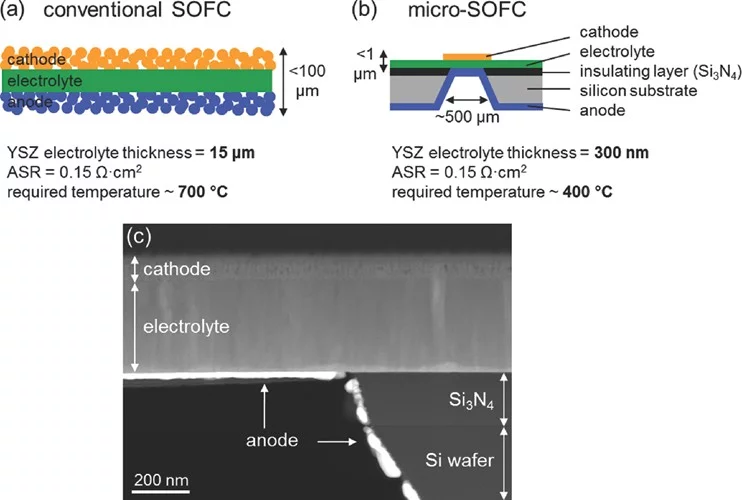
Partially amorphous La0.6Sr0.4CoO3-δ (LSC) thin-film cathodes are fabricated using pulsed laser deposition and are integrated in free-standing micro-solid oxide fuel cells (micro-SOFC) with a 3YSZ electrolyte and a Pt anode. A low degree of crystallinity of the LSC layers is achieved by taking advantage of the miniaturization of the cells, which permits low-temperature operation (300–450 °C). Thermomechanically stable micro-SOFC are obtained with strongly buckled electrolyte membranes. The nanoporous columnar microstructure of the LSC layers provides a large surface area for oxygen incorporation and is also believed to reduce the amount of stress at the cathode/electrolyte interface. With a high rate of failure-free micro-SOFC membranes, it is possible to avoid gas cross-over and open-circuit voltages of 1.06 V are attained. First power densities as high as 200–262 mW cm−2 at 400–450 °C are achieved. The area-specific resistance of the oxygen reduction reaction is lower than 0.3 Ω cm2 at 400 °C around the peak power density. These outstanding findings demonstrate that partially amorphous oxides are promising electrode candidates for the next-generation of solid oxide fuel cells working at low-temperatures.
Facility: ENE, ETH, LMX, Thin Films and Interfaces
Reference: Anna Evans, Julia Martynczuk, Dieter Stender, Christof W. Schneider, Thomas Lippert, and Michel Prestat , Adv. Energy Mater. 5, 1400747 (2015)
Read full article: here