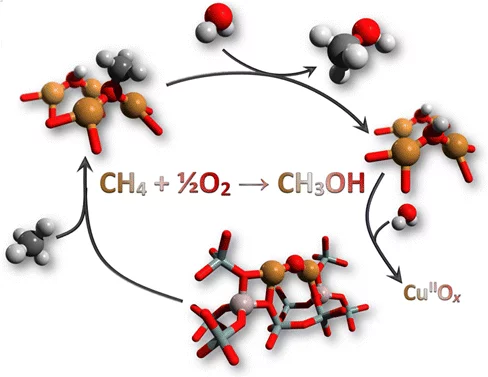
Determining the structure of the active Cu sites, which are associated with the methane conversion intermediate during stepwise, low-temperature, methane-to-methanol conversion, represents an important step for the upgrade of this reaction route to a viable process. Quick X-ray absorption spectroscopy allowed us to follow the electronic and structural changes to the active Cu sites during reaction with methane and during desorption of the activated intermediate. A large fraction (41%) of the oxygen-activated CuII reacted with methane and underwent reduction to CuI. When the intermediate was released as the product MeOH into the gas phase after reaction with water, the fraction of CuI was simultaneously converted back to CuII. Therefore, the activation of methane involves a change in the copper oxidation state. Density functional theory calculations identified [CuI−OCH3−CuII] and [CuI−OH−CuII] as energetically plausible structures of the adsorbed intermediates. The structure of the active Cu sites is also a function of conditions. In a dry pretreatment environment, the Cu sites took the form of dehydrated CuII oxide species, well characterized in the literature as mono(μ-oxo) and bis(μ-oxo)dicopper species. Under wet conditions, the dicopper species was destabilized to a hydrated CuII species, but a small amount of water-stable CuII oxide remained that was also active for conversion of methane to methanol.
Original Publication
Reaction Conditions of Methane-to-Methanol Conversion Affect the Structure of Active Copper SitesEvalyn Mae C. Alayon, Maarten Nachtegaal, Andras Bodi, Jeroen A. van Bokhoven
ACS Catalysis, Nov (2013)
DOI: 10.1021/cs400713c