Research Interests
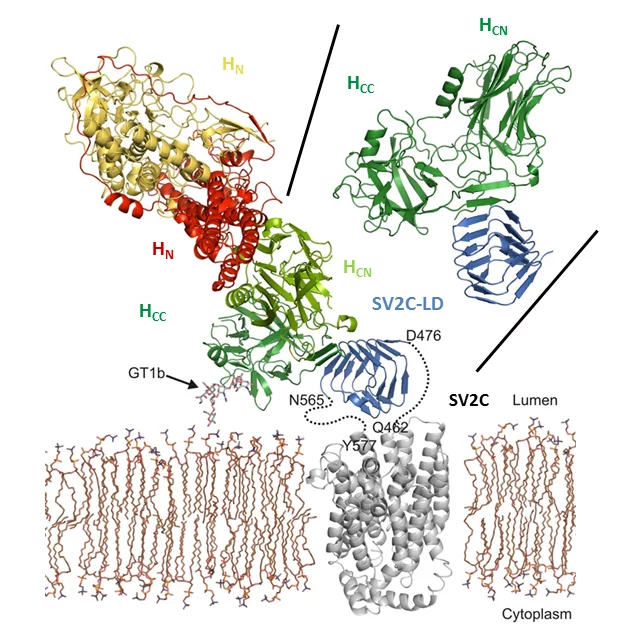
Botulinum neurotoxins (BoNTs) are among the most potent toxins known. They can cause botulism, a rare but potentially fatal paralytic disease and belong to the most dangerous bioweapons. Despite their toxicity, in particular BoNT/A1, commonly known as Botox, is used in cosmetic and medical applications. A detailed molecular understanding of BoNT/host protein interactions is fundamental both for developing strategies against botulism and for generating improved BoNT variants for medical applications.
Towards this aim, we are investigating toxin translocation and toxin/host receptor interactions. Furthermore, we are developing tools to study the structure and function of botulinum neurotoxins. To address these challenges, we use X-ray crystallography and cryo-electron microscopy (cryo-EM) in combination with biophysical, biochemical, and cell biological methods.
Key Publications
- Leka O, Wu Y, Li X, Kammerer RA. (2021) Crystal structure of the catalytic domain of botulinum neurotoxin subtype A3. J Biol Chem. 2021 Jan-Jun;296:100684. doi: 10.1016/j.jbc.2021.100684.
- Benoit RM, Schärer MA, Wieser MM, Li X, Frey D, Kammerer RA (2017) Crystal structure of the BoNT/A2 receptor-binding domain in complex with the luminal domain of its neuronal receptor SV2C. Sci Rep. 2017 Mar 2; 7:43588. doi: 10.1038/srep43588.
- Kammerer RA, Benoit RM (2014) Botulinum neurotoxins: new questions arising from structural biology. Trends Biochem Sci. 2014 Nov;39(11):517-26. doi: 10.1016/j.tibs.2014.08.009.
- Benoit RM, Frey D, Hilbert M, Kevenaar JT, Wieser MM, Stirnimann CU, McMillan D, Ceska T, Lebon F, Jaussi R, Steinmetz MO, Schertler GF, Hoogenraad CC, Capitani G, Kammerer RA (2014) Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature. 2014 Jan 2;505(7481):108-11. doi: 10.1038/nature12732.
Group Members
Publications since 2010
-
Nass KJ, Ilie IM, Saller MJ, Driessen AJM, Caflisch A, Kammerer RA, et al.
The role of the N-terminal amphipathic helix in bacterial YidC: insights from functional studies, the crystal structure and molecular dynamics simulations
Biochimica et Biophysica Acta: Biomembranes. 2022; 1864(3): 183825 (9 pp.). https://doi.org/10.1016/j.bbamem.2021.183825
DORA PSI -
Trebosc V, Lucchini V, Narwal M, Wicki B, Gartenmann S, Schellhorn B, et al.
Targeting virulence regulation to disarm Acinetobacter baumannii pathogenesis
Virulence. 2022; 13(1): 1868-1883. https://doi.org/10.1080/21505594.2022.2135273
DORA PSI -
Choo JPS, Kammerer RA, Li X, Li Z
High-level production of phenylacetaldehyde using fusion-tagged styrene oxide isomerase
Advanced Synthesis and Catalysis. 2021; 363(6): 1714-1721. https://doi.org/10.1002/adsc.202001500
DORA PSI -
Leka O, Wu Y, Li X, Kammerer RA
Crystal structure of the catalytic domain of botulinum neurotoxin subtype A3
Journal of Biological Chemistry. 2021; 296: 100684 (8 pp.). https://doi.org/10.1016/j.jbc.2021.100684
DORA PSI -
Fiedler T, Fabrice TN, Studer V, Vinet A, Faltova L, Kammerer RA, et al.
Homodimerization of coronin A through the C-terminal coiled-coil domain is essential for multicellular differentiation of Dictyostelium discoideum
FEBS Letters. 2020; 594(13): 2116-2127. https://doi.org/10.1002/1873-3468.13787
DORA PSI -
Li X, Brunner C, Wu Y, Leka O, Schneider G, Kammerer RA
Structural insights into the interaction of botulinum neurotoxin a with its neuronal receptor SV2C
Toxicon. 2020; 175: 36-43. https://doi.org/10.1016/j.toxicon.2019.11.010
DORA PSI -
Faltova L, Jiang K, Frey D, Wu Y, Capitani G, Prota AE, et al.
Crystal structure of a heterotetrameric katanin p60:p80 complex
Structure. 2019; 27(9): 1375-1383.e3. https://doi.org/10.1016/j.str.2019.07.002
DORA PSI -
Jiang K, Faltova L, Hua S, Capitani G, Prota AE, Landgraf C, et al.
Structural basis of formation of the microtubule minus-end-regulating CAMSAP-katanin complex
Structure. 2018; 26(3): 375-382. https://doi.org/10.1016/j.str.2017.12.017
DORA PSI -
Benoit RM, Schärer MA, Wieser MM, Li X, Frey D, Kammerer RA
Crystal structure of the BoNT/A2 receptor-binding domain in complex with the luminal domain of its neuronal receptor SV2C
Scientific Reports. 2017; 7: 43588 (7 pp.). https://doi.org/10.1038/srep43588
DORA PSI -
Burnett A, Gomez I, Davila De Leon D, Ariaans M, Progias P, Kammerer RA, et al.
Angiopoietin-1 enhances neutrophil chemotaxis in vitro and migration in vivo through interaction with CD18 and release of CCL4
Scientific Reports. 2017; 7(1): 2332 (9 pp.). https://doi.org/10.1038/s41598-017-02216-y
DORA PSI -
Jiang K, Rezabkova L, Hua S, Liu Q, Capitani G, Altelaar AFM, et al.
Microtubule minus-end regulation at spindle poles by an ASPM-katanin complex
Nature Cell Biology. 2017; 19(5): 480-492. https://doi.org/10.1038/ncb3511
DORA PSI -
Kaplan AR, Brady MR, Maciejewski MW, Kammerer RA, Alexandrescu AT
Nuclear magnetic resonance structures of GCN4p are largely conserved when ion pairs are disrupted at acidic pH but show a relaxation of the coiled coil superhelix
Biochemistry. 2017; 56(11): 1604-1619. https://doi.org/10.1021/acs.biochem.6b00634
DORA PSI -
Kumar A, Manatschal C, Rai A, Grigoriev I, Steiner Degen M, Jaussi R, et al.
Short linear sequence motif LxxPTPh targets diverse proteins to growing microtubule ends
Structure. 2017; 25(6): 924-932. https://doi.org/10.1016/j.str.2017.04.010
DORA PSI -
Rezabkova L, Jiang K, Capitani G, Prota AE, Akhmanova A, Steinmetz MO, et al.
Structural basis of katanin p60:p80 complex formation
Scientific Reports. 2017; 7: 14893 (8 pp.). https://doi.org/10.1038/s41598-017-14194-2
DORA PSI -
Vercellino I, Rezabkova L, Olieric V, Polyhach Y, Weinert T, Kammerer RA, et al.
Role of the nucleotidyl cyclase helical domain in catalytically active dimer formation
Proceedings of the National Academy of Sciences of the United States of America PNAS. 2017; 114(46): E9821-E9828. https://doi.org/10.1073/pnas.1712621114
DORA PSI -
Bianchi S, van Riel WE, Kraatz SHW, Olieric N, Frey D, Katrukha EA, et al.
Structural basis for misregulation of kinesin KIF21A autoinhibition by CFEOM1 disease mutations
Scientific Reports. 2016; 6: 30668 (16 pp.). https://doi.org/10.1038/srep30668
DORA PSI -
Hilbert M, Noga A, Frey D, Hamel V, Guichard P, Kraatz SHW, et al.
SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture
Nature Cell Biology. 2016; 18(4): 393-403. https://doi.org/10.1038/ncb3329
DORA PSI -
Rezabkova L, Kraatz SHW, Akhmanova A, Steinmetz MO, Kammerer RA
Biophysical and structural characterization of the centriolar protein CEP104 interaction network
Journal of Biological Chemistry. 2016; 291(35): 18496-18504. https://doi.org/10.1074/jbc.M116.739771
DORA PSI -
Sharma A, Aher A, Dynes NJ, Frey D, Katrukha EA, Jaussi R, et al.
Centriolar CPAP/SAS-4 imparts slow processive microtubule growth
Developmental Cell. 2016; 37(4): 362-376. https://doi.org/10.1016/j.devcel.2016.04.024
DORA PSI -
Benoit RM, Frey D, Wieser MM, Thieltges KM, Jaussi R, Capitani G, et al.
Structure of the BoNT/A1 - Receptor complex
Toxicon. 2015; 107(Part A): 25-31. https://doi.org/10.1016/j.toxicon.2015.08.002
DORA PSI -
Alfieri A, Ong ACM, Kammerer RA, Solanky T, Bate S, Tasab M, et al.
Angiopoietin-1 regulates microvascular reactivity and protects the microcirculation during acute endothelial dysfunction: role of eNOS and VE-cadherin
Pharmacological Research. 2014; 80: 43-51. https://doi.org/10.1016/j.phrs.2013.12.008
DORA PSI -
Benoit RM, Frey D, Hilbert M, Kevenaar JT, Wieser MM, Stirnimann CU, et al.
Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A
Nature. 2014; 505(7481): 108-111. https://doi.org/10.1038/nature12732
DORA PSI -
Jayachandran R, Liu X, BoseDasgupta S, Müller P, Zhang C-L, Moshous D, et al.
Coronin 1 regulates cognition and behavior through modulation of cAMP/Protein kinase A signaling
PLoS Biology. 2014; 12(3): e1001820 (21 pp.). https://doi.org/10.1371/journal.pbio.1001820
DORA PSI -
Kammerer RA, Benoit RM
Botulinum neurotoxins: new questions arising from structural biology
Trends in Biochemical Sciences. 2014; 39(11): 517-526. https://doi.org/10.1016/j.tibs.2014.08.009
DORA PSI -
Stroud MJ, Nazgiewicz A, McKenzie EA, Wang Y, Kammerer RA, Ballestrem C
GAS2-like proteins mediate communication between microtubules and actin through interactions with end-binding proteins
Journal of Cell Science. 2014; 127(12): 2672-2682. https://doi.org/10.1242/jcs.140558
DORA PSI -
Weber S, Stirnimann CU, Wieser M, Frey D, Meier R, Engelhardt S, et al.
A type IV translocated Legionella cysteine phytase counteracts intracellular growth restriction by phytate
Journal of Biological Chemistry. 2014; 289(49): 34175-34188. https://doi.org/10.1074/jbc.M114.592568
DORA PSI -
Bjelić S, Wieser M, Frey D, Stirnimann CU, Chance MR, Jaussi R, et al.
Structural basis for the oligomerization-state switch from a dimer to a trimer of an engineered cortexillin-1 coiled-coil variant
PLoS One. 2013; 8(5): e63370 (7 pp.). https://doi.org/10.1371/journal.pone.0063370
DORA PSI -
Holland JP, Kang A, Cohrs S, Selivanova SV, Milicevic Sephton S, Betzel T, et al.
Synthesis and evaluation of biphenyl compounds as kinesin spindle protein inhibitors
Chemistry and Biodiversity. 2013; 10(4): 538-555. https://doi.org/10.1002/cbdv.201200400
DORA PSI -
Prota AE, Magiera MM, Kuijpers M, Bargsten K, Frey D, Wieser M, et al.
Structural basis of tubulin tyrosination by tubulin tyrosine ligase
Journal of Cell Biology. 2013; 200(3): 259-270. https://doi.org/10.1083/jcb.201211017
DORA PSI -
Alfieri A, Watson JJ, Kammerer RA, Tasab M, Progias P, Reeves K, et al.
Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis
Critical Care. 2012; 16(5): R182 (12 pp.). https://doi.org/10.1186/cc11666
DORA PSI -
Alves-Silva J, Sánchez-Soriano N, Beaven R, Klein M, Parkin J, Millard TH, et al.
Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins)
Journal of Neuroscience. 2012; 32(27): 9143-9158. https://doi.org/10.1523/JNEUROSCI.0416-12.2012
DORA PSI -
Bjelić S, De Groot CO, Schärer MA, Jaussi R, Bargsten K, Salzmann M, et al.
Interaction of mammalian end binding proteins with CAP-Gly domains of CLIP-170 and p150glued
Journal of Structural Biology. 2012; 177(1): 160-167. https://doi.org/10.1016/j.jsb.2011.11.010
DORA PSI -
Beecher N, Roseman AM, Jowitt TA, Berry R, Troilo H, Kammerer RA, et al.
Collagen VI, conformation of A-domain arrays and microfibril architecture
Journal of Biological Chemistry. 2011; 286(46): 40266-40275. https://doi.org/10.1074/jbc.M111.265595
DORA PSI -
Stroud MJ, Kammerer RA, Ballestrem C
Characterization of G2L3 (GAS2-like 3), a new microtubule- and actin-binding protein related to spectraplakins
Journal of Biological Chemistry. 2011; 286(28): 24987-24995. https://doi.org/10.1074/jbc.M111.242263
DORA PSI -
Ciani B, Bjelić S, Honnappa S, Jawhari H, Jaussi R, Payapilly A, et al.
Molecular basis of coiled-coil oligomerization-state specificity
Proceedings of the National Academy of Sciences of the United States of America PNAS. 2010; 107(46): 19850-19855. https://doi.org/10.1073/pnas.1008502107
DORA PSI