Show filters
Advancing Biogas Quality: Tackling Siloxane Challenges for Smooth Energy Transition
Siloxanes, present in everyday items, can compromise the efficiency and durability of bioenergy systems, even at trace levels. Monitoring and quantifying these impurities are critical for improving biogas quality and expanding its role in renewable energy. However, sampling biogas and storing samples containing siloxanes for analysis remain a significant challenge.
How catalysts remove dangerous nitrogen oxides
In industrial catalysis, iron is not equal to iron.
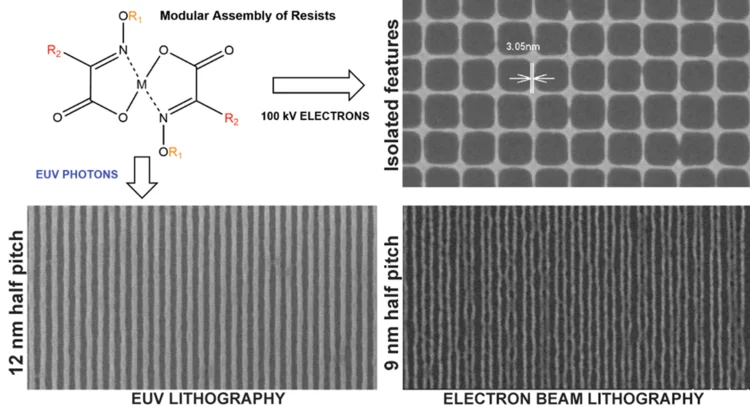
Novel Photoresist Chemistry Enables Lithography Approaching Angstrom-Scale Resolution
Photoresist materials are crucial in the manufacturing of computer chips, where the circuits are initially printed in the photoresist using photolithography. As the demand for smaller and more precise circuitry in computer chips grows, photoresists must resolve features with smaller sizes and higher density.
Revolutionizing Renewable Processes: Ambizione Grant Winner Emanuele Moioli
Emanuele Moioli is one of the recipients of the prestigious Ambizione Grant awarded by the Swiss National Science Foundation (SNSF) on a yearly basis. With the great news of his award having arrived in August 2022, Moioli embarks on the journey of his groundbreaking project titled “Moving catalyst vs. Multi-catalyst: determination of the best reactor for the processing of unconventional feedstock,” set to commence this August 2023.
A greener alternative for aviation fuel
Air travel with no carbon footprint – PSI and the Metafuels AG develop a new technology to produce sustainable aviation fuel.
Cooperation and licensing agreement between the Paul Scherrer Institute and Alphasynt
The Paul Scherrer Institute and Alphasynt have recently signed a cooperation- and licensing agreement. In doing so, the two pave the way for a fruitful collaboration in the commercialization of methanation.
Combining forces for the energy transition
The Paul Scherrer Institute PSI and the start-up AlphaSYNT are piloting a new approach for storing energy in the form of methane gas.
Power-to-gas system for energy independence
New technology provides synthetic natural gas for domestic heating
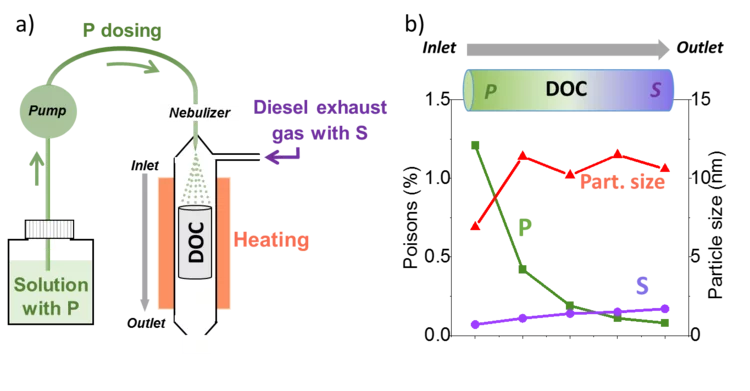
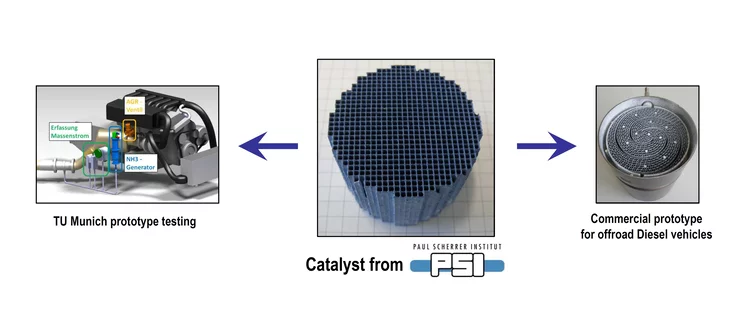
Unravelling the catalyst aging phenomena in vehicle emission control.
PSI has collaborated with catalyst and engine manufacturers to understand the aging phenomena of emission control catalysts. To this end, a diesel oxidation catalyst with a relevant mileage was carefully analysed; the results suggest that a complex combination of poisoning and thermal sintering is the cause of deactivation during driving. A reactor setup was then developed to simulate poisoning and sintering effects for prediction of catalyst durability in time and cost effective manner.
Getting maximum energy out of biomass
Researchers at the Paul Scherrer Institute PSI start operation of a revolutionary pilot plant for production of synthetic biogas.
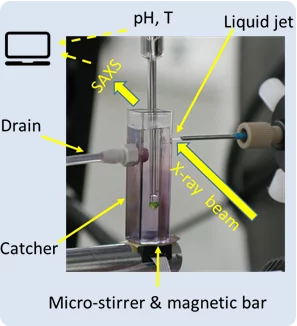
Matter before solid nucleation under synchrotron light
Investigation on early stage of solid formation from solution, before nucleation, have been carried out at PSI by small angle scattering technique using X-ray light from the Swiss Light Source SLS. The system under analysis was calcium carbonate, a model system archetype of several sparsely soluble inorganic materials and relevant in many field such as CO2 capturing and biomineralization. The experimental setup, the method, and developed theoretical framework can be applied to many other systems and made available for the entire scientific community.
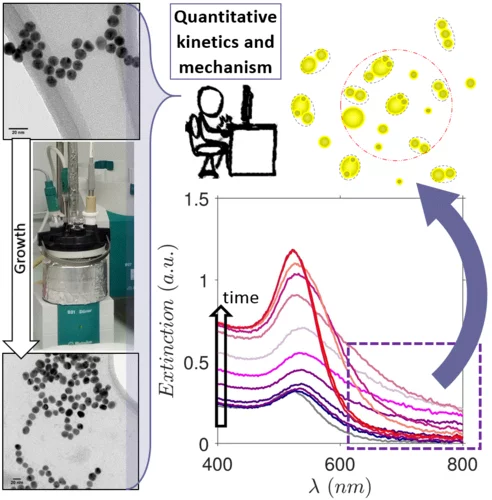
Kinetics and Mechanism of Metal Nanoparticle Growth via Optical Extinction Spectroscopy and Computational Modeling: The Curious Case of Colloidal Gold
An overarching computational framework unifying several optical theories to describe the temporal volution of gold nanoparticles (GNPs) during a seeded growth process is presented. To achieve this, we sed the inexpensive and widely available optical extinction spectroscopy, to obtain quantitative kinetic data. In situ spectra collected over a wide set of experimental conditions were regressed using the hysical model, calculating light extinction by ensembles of GNPs during the growth process. This model rovides temporal information on the size, shape, and concentration of the particles, and any electromagnetic interactions between them. Consequently, we were able to describe the mechanism of GNP growth and divide the process into distinct genesis periods. We provide explanations for several longstanding mysteries, e.g., the phenomena responsible for the purple-greyish hue during the early stages of GNP growth, the complex interactions between nucleation, growth and aggregation events, and a clear distinction between agglomeration and electromagnetic interactions. The presented theoretical formalism has been developed in a generic fashion so that it can readily be adapted to other nanoparticulate formation scenarios such as the genesis of various metal nanoparticles.
Cleaner emissions thanks to sponge-like structure
PSI researchers have developed a new catalytic converter for cleaning emissions from natural gas engines. It is very active even at low temperatures and remains that way over a long period of time. This allows natural gas to be burned in a cleaner, more climate-friendly way. Thus natural gas and biogas become more attractive as substitutes for petroleum products – for example, as fuel for cars.
Supported gold as catalyst for the decomposition of ammonia precursors in the selective catalytic reduction of NOx
Titaniumdioxide supported gold was found to catalyze the hydrolysis of formate-based ammonia precursor compounds which are proposed for the selective catalytic reduction of nitrogen oxides (NOx) in combustion exhaust gas. In contrast to other noble metals, the supported gold does not oxidize the released NH3, while it maintains decomposition of intermediate formic acid.
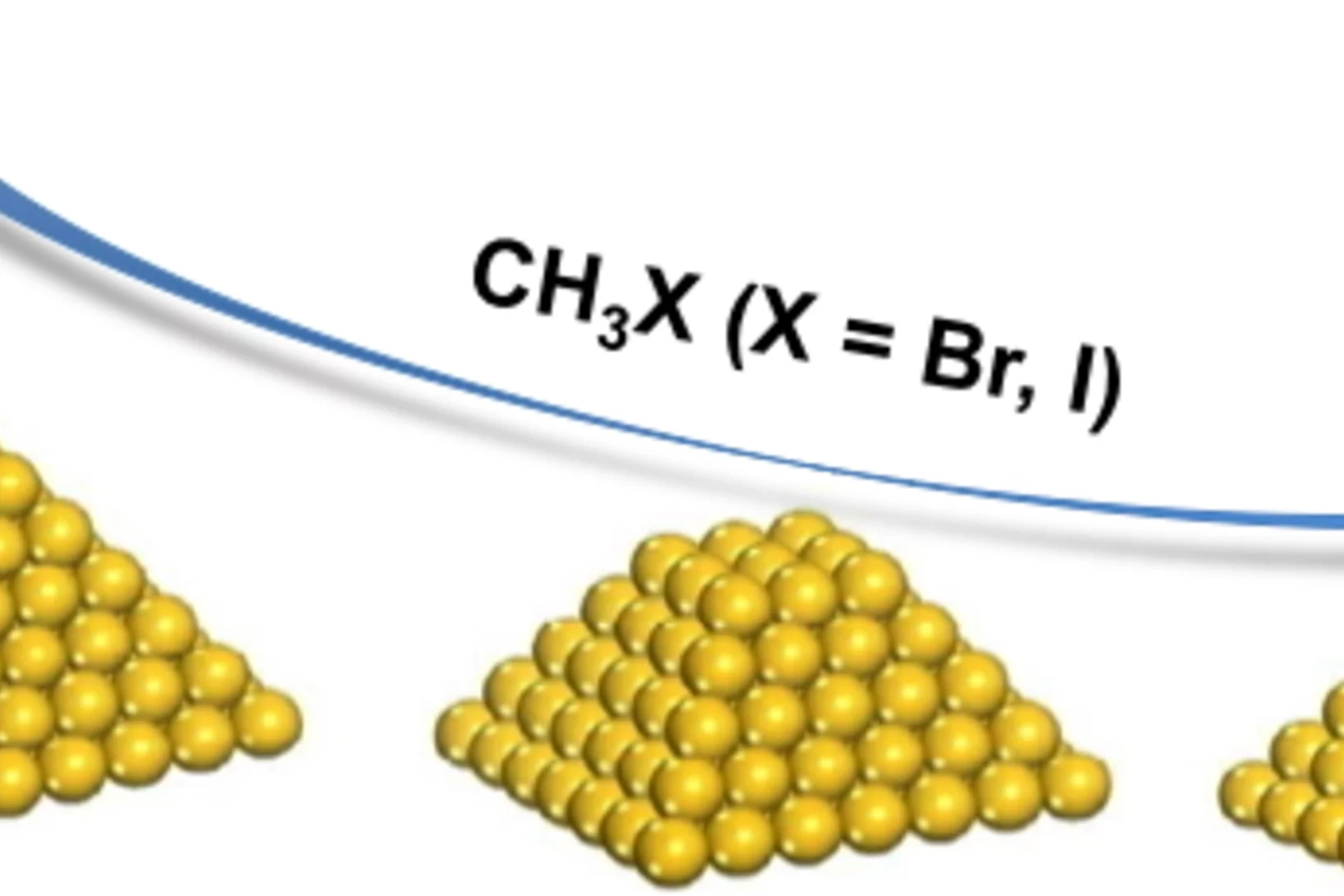
Influence of Methyl Halide Treatment on Gold Nanoparticles Supported on Activated Carbon
Gold particles supported on carbon when subjected to a flow of methyl iodide or bromide redisperse from large ensembles to single atoms and/or dimers of gold. Methyl halide oxidizes gold leading to gradual particle dissolution. The process could be carried out at temperatures as low as 50 °C. The excess of halide could be removed by a post-treatment of the material with 1%H2O/H2, which does not influence the metal dispersion. This remarkable transformation opens the possibility of re-activating gold catalysts that lost their performance due to metal particles sintering.
Vibrational Spectra of Adsorbates from DFT
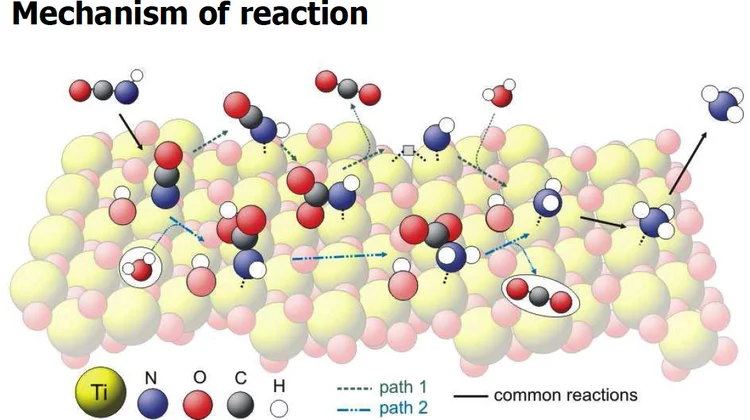
The hydrolysis of isocyanic acid was studied experimentally and theoretically and a reaction mechanism on different catalysts was established. The decreasing NOx emission limits for diesel vehicles impel the further development of the existing NOx deactivation technologies, particularly the selective catalytic reduction (SCR) of nitrogen oxides with urea. In the urea-SCR process, urea is injected into the hot exhaust gas, where it thermally decomposes into isocyanic acid (HNCO) and ammonia.